Urinary Tract Infections
Carol E. Chenoweth
Urinary tract infection (UTI) is one of the most frequently reported healthcare-associated infections (HAIs), accounting for up to 40% of all HAIs (1,2,3). Most healthcare-associated UTIs (70%) are associated with urinary catheters; in intensive care units (ICUs), as many as 95% of all UTIs are associated with urinary catheters (4,5). Urinary catheters are widely used in healthcare today, especially in ICUs, in long-term care facilities, and increasingly in home care patients (4,6,7). Up to 25% of patients have a urinary catheter placed at some time during their hospital stay (8,9). The Centers for Disease Control and Prevention (CDC) estimates that 139,000 catheter-associated UTIs (CA-UTIs) occurred in US hospitals in 2007 (4).
CA-UTIs are associated with increased morbidity, mortality, and costs. CA-UTIs are associated with excess mortality, even after controlling for severity of illness and other underlying comorbidities. More significantly, healthcare-associated bloodstream infection originating from a urinary source has a case-fatality rate of 32.8% (10,11). Each episode of CA-UTI is estimated to cost approximately $600; if associated with a bloodstream infection, costs increase to at least $2,800 (12). Nationally, CA-UTIs result in as much as $131 million annual excess medical costs.
Since October 2008, the Centers for Medicare and Medicaid Services (CMS) no longer reimburses for costs associated with treatment of hospital-acquired CA-UTIs (12). Consequently, prevention of CA-UTIs has become a priority for most hospitals, as 65% to 70% of CA-UTIs are potentially preventable (13). This chapter reviews the pathogenesis, epidemiology, and preventive measures for CA-UTIs.
PATHOGENESIS
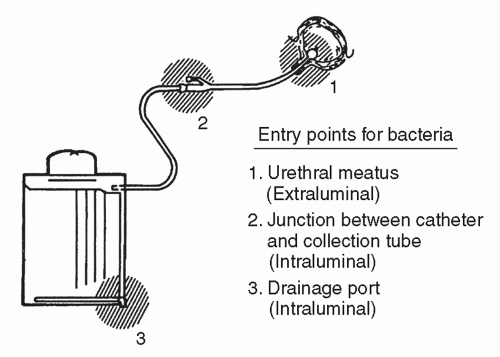
Urinary catheters readily develop a biofilm composed of clusters of microbial organisms on the internal and external catheter surface surrounded by an extracellular matrix made up of primarily polysaccharide materials (2,14,15,16,17). The biofilm allows for microbial attachment and adherence to catheter surfaces. The microorganisms gain access to the catheter and attach to the biofilm via one of two routes: extraluminally or intraluminally (Figure 31.1). Extraluminal organisms are primarily endogenous, originating from the patient’s gastrointestinal tract and colonizing the patient’s perineum. The organisms ascend the catheter by direct inoculation at the time of catheter insertion or by migration in the mucous sheath surrounding the external aspect of the catheter (2,18). Approximately 70% of episodes of bacteriuria in catheterized women are felt to occur through extraluminal entry of organisms (18). In a recent prospective study of 173 CA-UTIs, 115 (66%) were likely acquired through the extraluminal route (18).
The microorganisms also enter the catheter intraluminally when organisms gain access to the internal lumen of the catheter through failure of a closed drainage system or contamination of the collection bag (2,18,19). These organisms, usually introduced from exogenous sources, often are the result of cross-transmission of organisms on the hands of healthcare workers (HCWs) (2,19,20). Intraluminal contamination of the collecting system was recently found to account for 34% of CA-UTIs (18). Once the microorganisms attach and multiply, the resultant sheet of organisms secretes an extracellular matrix of bacterial glycocalyces, imbedding the microorganisms (2,14,15,16,17,21).
Bacteria within the biofilm grow much more slowly than planktonic bacteria and secrete chemical signals that mediate population density-dependent gene expression (2,14,15,16,17,21). The migration of the biofilm over the inner surface of the catheter to the bladder occurs within 1 to 3 days or more quickly by swarming organisms, such as Proteus mirabilis (15,16,21). Most biofilms are composed of single organisms; however, biofilms may contain a mixture of up to five organisms (2,22). Some organisms, such as Providencia stuartii, Pseudomonas sp., enterococci, or Proteus sp., persist in the urine for up to 10 weeks, while other organisms appear to spontaneously cycle in and out (2,22). Several studies suggest that planktonic bacteria found in cultures obtained from the catheter may not reflect the bacterial population growing within the biofilm (2,22). Proteus sp., Pseudomonas aeruginosa, Klebsiella pneumoniae, and Providencia sp. have the ability to hydrolyze urea in the urine to free ammonia. The resulting increase in local pH allows precipitation of minerals such as hydroxyapatite or struvite. Mineral deposition within the catheter biofilm causes encrustations that are unique to biofilms formed on urinary catheters (21,23). Encrustations
on the inner surface of the catheter can build to completely block catheter flow or act as a nidus for the formation of renal calculi (24,25).
on the inner surface of the catheter can build to completely block catheter flow or act as a nidus for the formation of renal calculi (24,25).
The urinary biofilm provides a protective environment from the activity of antimicrobial agents (26,27). First, the extracellular matrix may prevent the penetration of antimicrobials into the biofilm. For example, both ciprofloxacin and tobramycin have poor diffusion into biofilms. Second, organisms growing at a slower rate within the biofilm are more resistant to the effects of antimicrobial agents that require active growth (23,26,27). Finally, chemical signaling from organisms growing within the biofilm appears to regulate the genes that alter the molecular targets of antimicrobials (23). The features of the biofilm as described have important implications for both prevention and treatment of CA-UTIs.
EPIDEMIOLOGY
MICROBIAL ETIOLOGY
Enterobacteriaceae, including Escherichia coli, and Klebsiella sp., are the most common pathogens associated with CA-UTIs (Table 31.1). Other pathogens, more common in the ICU setting, include P. aeruginosa, enterococci, and Candida sp. (1,11,28). European hospitals report a similar spectrum of bacteria associated with healthcare-associated UTIs, except for Pseudomonas sp., which were isolated in only 7% of urine cultures (29). In data reported from the CDC’s National Healthcare Safety Network (NHSN) in 2009 to 2010, 29.1% and 33.5% of E. coli CA-UTI isolates from patients in an ICU or non-ICU settings, respectively, were resistant to fluoroquinolones (30). In addition, 24.6% to 29.0% of Klebsiella sp. and 11.5% to 13.2% of E. coli isolates from patients with CA-UTIs produced extended-spectrum beta-lactamases. Even more concerning is that during this same time period, 15.2% to 17.0% of all Klebsiella sp. from patients with CA-UTIs were resistant to carbapenems (30). Long-term acute care hospitals (LTACHs) have a prevalence of resistant Enterobacteriaceae in CA-UTI isolates that is similar to that seen in ICUs in acute care hospitals (28).
Enterococci emerged as a significant cause of healthcare-associated UTIs between 1975 and 1984 (31,32). Enterococcal UTIs may derive from an endogenous source such as the patient’s fecal flora or may be acquired exogenously (33,34,35). The emergence and spread of vancomycin-resistant strains have compounded the problem in acute care hospitals and LTACHs (28,30). Candida spp. are prevalent in the medical ICU setting, where 28% of UTI are associated with Candida spp. (28). The risk factors for candiduria include prolonged catheterization and use of broad-spectrum antimicrobials (36). Coagulase-positive staphylococci (CPS) are an infrequent cause of CA-UTI (1,11), but when CPS-UTIs occur, secondary bacteremia may result. Conversely, CPS bacteremia or endocarditis may result in secondary infection of the urinary tract. In one study, 27% of CPS bacteremias were associated with secondary bacteriuria (37). The presence of CPS in the urine should prompt consideration for coinciding bacteremia or endocarditis (37,38).
While 80% of infections associated with short-term indwelling urinary catheters are caused by single organisms, infections in long-term catheters are frequently polymicrobial. UTIs in long-term catheters are associated with ≥2 organisms in 77% to 95% of episodes, and 10% have >5 species of organisms present (1,3,39).
TABLE 31.1 Microbial Pathogens Associated with Catheter-Related Urinary | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
INCIDENCE OF CATHETER-ASSOCIATED UTI
In hospital-wide data, UTIs have accounted for ˜40% of all HAIs, but UTIs make up a smaller proportion of HAIs occurring in the ICU setting (1,5). With widespread interventions occurring nationwide, the rates of CA-UTI in ICUs have decreased significantly between 1990 and 2007 (4). In 2010, the rates of CA-UTIs reported to CDC’s NHSN ranged from 4.7/1,000 catheter-days in burn ICUs to 1.3/1,000 catheter-days in medical/surgical ICUs. The rates of CA-UTI in pediatric ICUs were reported at 2.2 to 3.9/1,000 catheter-days (40); however, CA-UTI is infrequently identified in neonatal ICUs (41). Surprisingly, general care wards report rates equivalent to or higher than ICU settings, with a range from 0.2 to 3.2/1,000 catheter-days. Among general care wards, rehabilitation units have the highest rates of CA-UTI (5,40).
RISK FACTORS FOR CATHETER-ASSOCIATED UTI
The most important and consistent risk factor for bacteriuria is the duration of urinary catheterization (odds ratio [OR] = 2.3 to 22.4, depending on duration) (42,43,44). Urinary catheters are associated with the vast majority of healthcare-associated UTIs; up to 95% of UTIs in ICUs are associated with a urinary catheter (1,5). Bacteriuria occurs quickly and frequently in catheterized patients with an average daily risk of 3% to 10% per day. In patients with a catheter indwelling for 2 to 10 days, 26% will develop bacteriuria (45,46,47). Nearly all patients catheterized for a month will have bacteriuria, making this the dividing line between short-term and long-term catheterization (2,22).
Females have a higher risk of bacteriuria than males (relative risk [RR] = 1.7 to 3.7) (42,43,44,45). Systemic antibiotics have a protective effect on bacteriuria; therefore, the absence of systemic antimicrobials increases the risk of bacteriuria (RR = 2.0 to 3.9) (42,43,44,45). Nonadherence to catheter care recommendations has been associated with increased risk of bacteriuria (19,42,43). Other risk factors identified in ≥1 studies include rapidly fatal underlying illness (RR = 2.5) (42); age >50 years (RR = 2) (42); nonsurgical disease (RR = 2.2) (42); hospitalization on an orthopedic (RR = 51) or urological service (RR = 4) (48); catheter insertion after the sixth day of hospitalization (RR = 8.6) (48); catheter inserted outside the operating room (RR = 5.3) (43); diabetes mellitus (OR = 2.3) (43);
or serum creatinine >2 mg/dL at the time of catheterization (OR = 2.1) (43). Heavy periurethral colonization with bacteria also has been associated with increased risk of bacteriuria (49). The significant risk factors for CA-UTI are summarized in Table 31.2.
or serum creatinine >2 mg/dL at the time of catheterization (OR = 2.1) (43). Heavy periurethral colonization with bacteria also has been associated with increased risk of bacteriuria (49). The significant risk factors for CA-UTI are summarized in Table 31.2.
TABLE 31.2 Risk Factors Associated with the Development of Catheter-Associated Bacteriuria | ||
|---|---|---|
|
UTI-related bloodstream infections (BSIs) occur infrequently (<4% of patients with catheter-related bacteriuria develop bacteremia) (10,50,51,52). In an early prospective study by Krieger et al., BSIs from a urinary tract origin were found in 2.6% of patients with UTIs (51). A more recent study reported a rate of 1.4 BSIs from a urinary tract source per 10,000 patient-days (53). In a case series from an academic medical center, Enterococcus sp. (28.7%) and Candida sp. (19.6%) were the predominant microorganisms associated with hospital-acquired BSI from a urinary source (10). The risk factors for secondary healthcare-associated BSI identified in multiple studies include UTI due to Serratia marcescens, compared with other organisms (RR = 3.5), male gender, immunosuppression, cigarette use, number of hospital-days before bacteriuria, neutropenia, and renal disease (50,51,54).
CLINICAL MANIFESTATIONS
CA-UTI presents clinically with a spectrum from asymptomatic bacteriuria to urosepsis and death (1,2,55,56). Only 10% to 32% of patients with catheter-associated bacteriuria experience symptoms attributable to infection; thus, most patients can be classified as having asymptomatic bacteriuria (1,2,55,56




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree