Major Concepts
History
Infectious disease agents have been harnessed for centuries as weapons to neutralize armies, decrease the size of enemy populations, and create a state of terror that destabilizes societies. Such biological weapons research continued in many “civilized” nations until very recently, and clandestine work may be ongoing despite being banned by international treaty. Even though many known stocks of bioweapons have “disappeared,” government groups around the world continue to prepare for attacks that hopefully will never occur.
Potential Bioweapon Agents and Diseases
Several species of bacteria and viruses and some bacterial toxins are perceived to be of potential use by bioterrorist groups. The agents believed to pose the greatest threat to humans are Bacillus anthracis (anthrax), Yersinia pestis (plague), Francisella tularensis (tularemia), Variola major (smallpox), hemorrhagic fever viruses, and Clostridium botulinum toxin (botulism). Many of these agents have been released intentionally. Other bacteria and viruses have also been the subject of research as agents of biological warfare. In addition to microbes that target humans, agents that kill animals have been studied for use in crippling a region’s food supply or economy.
Preparation for an Attack
Governmental and public health organizations have ongoing plans for the early detection of and response to biological attacks. Researchers continue to prepare stocks of protective vaccines and antibiotics. Terrorist groups, however, have engineered bacteria and viruses in ways that increase their pathogenicity and allow them to circumvent the actions of current antibiotics and vaccines. The need to maintain active surveillance programs and to develop novel means of protection against genetically altered organisms consumes resources that might otherwise be used for more productive and beneficial projects.
A number of biological agents have been judged to pose serious risks to human populations if used in a biological attack. Such agents possess several of the following characteristics:
- High morbidity and mortality rates
- Potential for person-to-person transmission
- Low infective dose
- High infectivity following aerosol dissemination by long-range missiles
- Ability to cause large outbreaks
- Lack of an effective vaccine (either not yet developed or available in limited quantities)
- Potential to cause public and health care worker anxiety and disrupt social stability
- Terrorist group access to the microbe or toxin
- Feasibility of large-scale production
- Stability in storage and in the environment
- Prior research and development as a weapons agent
Particular microbes or toxins are further favored if their release is difficult to detect (silent, invisible, odorless, tasteless), if they induce diseases that are difficult to diagnose, and if they have been engineered to contain pathogenic elements of several agents or to be resistant to currently used vaccines and antibiotics. Agents whose production requires the use of skilled workers or specialized equipment and those with a high risk of infecting production workers or the population of the surrounding region are less useful.
The agents have been divided into three categories, A, B, and C, with category A agents deemed to pose the greatest threat. Category A agents are those that are easily disseminated or transmitted from person to person, have a high mortality rate with the potential for major public health impact, would cause public panic and social disruption, and require special actions for public health preparedness. Category B agents are those that are moderately easy to disseminate, are associated with a moderate morbidity and a low mortality rate, and require specific enhancement of diagnostic capacity and enhanced disease surveillance. Category C includes emerging infectious agents with potential to be engineered for mass dissemination in the future due to their availability, ease of production and dissemination, potential for high morbidity and mortality, and major impact on health.
Table 30.1 Categories of potential biological weapons agents
| Biological Agent | Disease | Infective Dose |
| Category A | ||
| Bacillus anthracis | Anthrax | 8,000–50,000 organisms |
| Yersinia pestis | Plague | 100–500 |
| Francisella tularensis | Tularemia | 10–50 |
| Variola major | Smallpox | 10–100 |
| Hemorrhagic fever viruses | Hemorrhagic fever | 1–10 |
| Clostridium botulinum toxin | Botulism | 0.001 μg/kg body weight (via intravenous, subcutaneous, or intraperitoneal inoculation); 0.003 μg/kg body weight (via inhalation) |
| Category B | ||
| Brucella species | Brucellosis | 10–100 bacteria |
| Burkholderia mallei | Glanders | Low |
| Coxiella burnetii | Q fever | 1–10 |
| Encephalomyelitis viruses | Viral encephalomyelitis | 1–100 |
| Ricinus communis toxin | Ricin intoxication | 1 molecule per cell |
| Category C | ||
| Multidrug-resistant Mycobacterium tuberculosi | Tuberculosis | N.D. |
| Other encephalomyelitis viruses | Viral encephalomyelitis | N.D. |
Note: N.D. = not yet determined.
The Black Death (bubonic plague), which killed approximately one-third of the world’s population, is believed to have originated in China in the 1330s and to have entered Europe in 1346 during a battle for the seaport city of Caffa on the Crimean Sea (currently part of Ukraine). Tartar forces catapulted plague victims into the city to spread the disease. Genoese merchants escaped Caffa in ships that later docked in Genoa, Italy, and spread the disease to Mediterranean ports through infected rats that escaped the ships. Plague then disseminated rapidly throughout Europe, claiming large numbers of victims and eventually destroying the feudal system in western Europe due to the fact that so many of the peasants who had formerly worked the land had died. Outbreaks continued to sweep the region from the fifteenth to eighteenth centuries.
Bubonic plague continued to be exploited as a bioweapon for centuries. During the battle of Carolstein in 1422 and the battle of Reval in 1710, Lithuanian and Russian forces also catapulted bodies of plague victims into the ranks of enemy troops. Variola major was much later rumored to have been used in the eighteenth century by the British, who gave clothing contaminated by smallpox scabs to Native Americans in order to initiate epidemics that would weaken the tribes to allow expansion of British colonies. During World War II, the Japanese attempted to develop smallpox for use in Mongolia and China.
A number of nations have performed basic research on and production of bioweapons in the recent past. These activities may be ongoing in some regions of the world. The U.S. offensive biological weapons program was officially ended in 1969–1970 by executive order of President Richard Nixon. The British program had ended much earlier, during the 1950s. The 1973 worldwide Biological and Toxin Weapons Convention banned development, production, stockpiling, and acquisition of biological weapons and was supposedly implemented in 1975. No effective international mechanism was set in place, however, for challenging development of these weapons. The number of countries engaged in such endeavors reportedly doubled over the course of the following decade.
In the Soviet Union, the bioweapons research and development program actually grew in size and scope from 1972 to 1987 under the auspices of several secret groups, including the Fifteenth Main Directorate of the Ministry of Defense and the “civilian” pharmaceutical complex Bioprepara. Microbial agents were genetically engineered for greater pathogenicity, to escape protective effects of the vaccines in use at the time, or to be resistant to antibiotics. Large stockpiles of plague and smallpox were prepared for delivery to large population centers using intercontinental ballistic missiles. Iraq also continued to run an active bioweapons production program.
Bioterrorism Agents and Diseases
Bacterial Diseases
Pneumonic Plague and Yersinia pestis
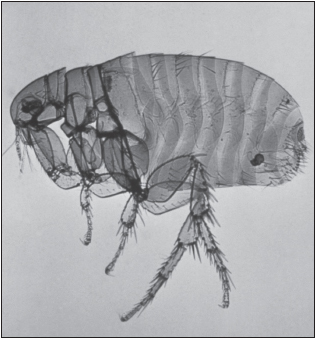
Yersinia pestis is a nonmotile gram-negative coccobacillus that serves as the causative agents of the three forms of plague in humans: bubonic, septicemic, and pneumonic. Y. pestis typically infects humans through the bite of an infected rat flea. Plague is an enzootic infection of rats, ground squirrels, prairie dogs, and other rodents in most continents, including North America, where it is found in the southwestern United States. The reservoir hosts often experience a large population die-off prior to an outbreak in humans. Most of the persons infected develop bubonic plague, with a sudden onset of fever, chills, and weakness and production of buboes—swollen, reddened, extremely tender lymph nodes in the groin, axillae, or neck.
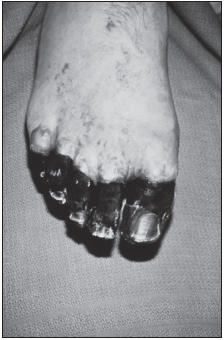
Some persons develop primary septicemic plague in which septicemia (blood infection) occurs directly after the flea bite in the absence of buboes. Secondary septicemia may also appear following bubonic plague. Septicemic plague is characterized by disseminated intravascular coagulation, necrosis of small vessels, purpuric skin lesions, and gangrene in the digits or nose during advanced disease. Neither bubonic nor septicemic plague is transmitted via direct person-to-person contact.
Symptoms of pneumonic plague include severe bronchopneumonia, chest pain, dyspnea, cough, and bloody sputum. It may develop as a secondary illness in a small number of patients with bubonic plague as bacilli spread from the blood into the lungs or as a primary infection. During primary pneumonic plague, pneumonic symptoms are accompanied by gastrointestinal symptoms such as nausea, vomiting, abdominal pain, and diarrhea. Pneumonic plague may be spread between people via inhalation of respiratory droplets, leading to primary pneumonic plague. Of the 390 cases of plague reported in the United States from 1947 to 1996, 84% were bubonic, 13% were septicemic, and 2% were pneumonic, with fatality rates of 14%, 22%, and 57%, respectively.
Pneumonic plague has received much attention for use in biological warfare. During World War II on at least three occasions, Unit 731 of the Japanese army is believed to have delivered a mixture of rice, wheat, and plague-infected fleas (Pulex irritans) to populated areas of China, leading to outbreaks of plague. In a twist of fate, cases of plague occurred in Japanese soldiers following a biological attack on Changde in 1941, resulting in 1,700 Japanese deaths.
The United States and the Soviet Union spent years perfecting this weapon by aerosolizing plague directly. A 1970 WHO study concluded that in a worst-case scenario, 50 kilograms of Y. pestis released as an aerosol over a city of 5 million could produce up to 150,000 cases of pneumonic plague and 36,000 deaths. Such an attack would differ from naturally occurring plague in that inhalation of aerosolized Y. pestis would cause rapidly progressive primary pneumonic plague one to six days from the time of exposure. A high death rate from septic shock would be postulated to occur without early treatment using streptomycin, gentamycin, or members of the tetracycline or fluoroquinolone classes of antibiotics. These drugs could also be used for prophylactic purposes.
Pulmonary Anthrax and Bacillus anthracis
B. anthracis is a gram-positive spore-forming bacillus that infects cattle, sheep, and horses in addition to humans. The bacteria are currently found among both wild and domestic animals in Asia, Africa, South and Central America, eastern and southern Europe, the Caribbean, and the Middle East. The hardy spores resist dehydration and other adverse environmental conditions to persist in the soil of pastures for years (up to 200 years, according to some reports). B. anthracis produces the highly pathogenic anthrax toxin, encoded by a plasmid. It is composed of three synergistically acting proteins: edema factor, lethal factor, and protective antigen. B. anthracis has been postulated to be a genetic variant of the nonpathogenic B. cereus and B. thuringiensis, which acquired plasmids that produce the toxin and a protective capsule.
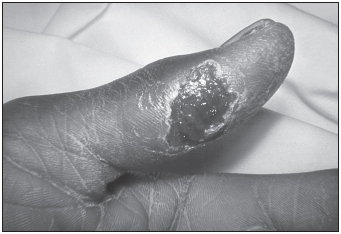
Disease manifestations take three forms: cutaneous, gastrointestinal, and inhalational. Cutaneous anthrax is the most common. An ulcerative skin lesion is produced following exposure of the skin to spores. The lesion later transforms into a black scab containing a necrotic core surrounded by blood-stained fluid and edema prior to self-resolution. Gastrointestinal anthrax is very rare and is acquired by ingesting undercooked meat contaminated by spores. Inhalational (pulmonary) anthrax is the form that bioterrorists seek to induce. This form is acquired by inhaling 8,000 to 50,000 aerosolized spores. After the bacteria are ingested by macrophages in the lungs, they are transported to regional lymph nodes, where they germinate, divide, and begin to produce toxin. Several days of malaise, mild fever, and cough are followed by progressive respiratory distress, cyanosis with massive edema in the neck and chest, and elevated pulse, respiration, and body temperature. Large numbers of bacteria are present in the lungs, blood, and other tissues. Local necrosis and edema may compress respiratory passages, and the toxin may depress the respiratory centers of the brain. This form of disease is rapidly fatal if untreated due to thrombosis of the pulmonary capillaries. The mortality rate is 95% when therapy is initiated longer than 48 hours after the beginning of symptomatic disease. Fortunately, several antibiotics are very effective against all forms of anthrax infection if administered early.
The use of anthrax as a bioweapon began early in the twentieth century as Germany infected livestock during World War I. In 1941, Britain tested the effect of anthrax on sheep on the island of Gruinard in Scotland. Decontamination proved very difficult, and the island was not considered safe until 1990. During World War II, Unit 731 of the Japanese army stockpiled 400 kilograms of anthrax spores for use in bombs. The United States and the Soviet Union as well as other nations developed anthrax as a bioweapon in the 1950s and 1960s. An accidental release of spores occurred from a Soviet weapons facility in Sverdlovsk in April 1979. During this outbreak of inhalational anthrax, 68 of 79 victims died and animal infections were reported more than 50 kilometers from the facility. The Soviet government at the time claimed that the fatalities resulted from gastrointestinal anthrax contracted by ingestion of infected meat. In 1992, President Yeltsin revealed the involvement of a military facility in this outbreak.
Several attacks with anthrax spores have occurred since 1990. In 1993, the Japanese Aum Shinrikyo terrorist cult released aerosolized spores from the top of a building in Kameido, a city near Tokyo. Fortunately, they used a nonpathogenic strain, Sterne 34F2, which is usually used for vaccination. An unknown group was responsible for a moderately successful series of anthrax attacks in the United States in September and October 2001, not long after the destruction of the World Trade Center in New York City. Five letters were sent containing anthrax spores. The first two were sent to NBC Television in New York and the New York Post. These contained only 10% spores but used the Ames strain, one of the most virulent strains of naturally occurring B. anthracis. Several people were sickened as a result of exposure. The other three letters were sent to a Florida tabloid newspaper, The Sun, and to the offices of U.S. Senators Patrick Leahy and Tom Daschle. These letters were far more sophisticated and contained pure 10-micrometer-sized spores that had been chemically stabilized. Five people died during this attack, and two dozen more were infected, several of these people being exposed at a post office after handling the sealed letters.
One of the difficulties in the weaponization of anthrax is production of the most effective spores. Spores must be of a proper size to stay airborne until inhaled and must be able to pass into the lower reaches of the respiratory tree to inflict the most harm to the greatest number of individuals. Ten micrometers is believed to be the optimal size for deep lung penetration. The anthrax letter attack in the United States may have produced this size of particles by spray-drying. Weaponized B. anthracis has often been genetically modified so as to be resistant to many antibiotics.
Tularemia (Rabbit Fever) and Francisella tularensis
F. tularensis is a small, nonmotile, aerobic, gram-negative coccobacillus with a thin lipopolysaccharide-containing envelope. These bacilli are facultative intracellular bacteria that multiply within macrophages, targeting the lymph nodes, lungs, spleen, liver, and kidneys. Hardy and non-spore-forming, these bacteria are able to survive for weeks at low temperatures in water, moist soil, hay, straw, and decaying animal carcasses.
Tularemia is common in humans in parts of Europe, including Sweden, Finland, Spain, and Kosovo. It is also present in North America (1,368 cases were reported in the United States during the 1990s). Two major subspecies (biovars) exist, differing in virulence and epidemiological features. Biovar tularensis (type A) is the most common type in North America and is highly virulent in humans and animals. Biovar palaearctica (holarctic; type B) is most common in Europe and Asia and is relatively avirulent. Bacteria may enter humans through the skin, mucous membranes, gastrointestinal tract, and lungs; following bites by infective arthropods (ticks, deerflies, or mosquitoes); handling infected animal tissues; contact with or ingestion of contaminated water, food, or soil; or inhalation of infective aerosols generated by a lawn mower or brush cutter. Wild animals, including rabbits, squirrels, muskrats, beaver, and deer, may be infected, as may, less commonly, domestic animals such as sheep, cats, and dogs.
Infection with F. tularensis may take several forms. Respiratory tularemia (tularemia pneumonia) is the form that would follow a biological attack; it results from inhalation of bacteria-laden aerosols. Onset of disease is abrupt and is characterized by fever, headache, chills and rigor, generalized body ache, and sore throat followed by profound sweating, progressive weakness, anorexia, and weight loss, all of which may become incapacitating within a day or two. Symptoms of pulmonary infection include dry cough and substernal pain or tightness in the presence or absence of objective signs of bronchopneumonia, including purulent sputum, difficulty breathing, and rapid breathing rate. Hemorrhagic airway inflammation may occur as alveoli fill with exudates of mononuclear cells, leading to inflammation of the pleural membranes and hilar lymphadenopathy that rapidly progresses to severe pneumonia, respiratory failure, and death. Untreated, symptoms may remain for weeks to months and may be spread via the circulatory system, resulting in secondary pneumonia, sepsis, and occasionally meningitis. Tularemia sepsis is severe and may be fatal. It is characterized by fever, abdominal pain, diarrhea, and vomiting early after infection. If not treated promptly, affected persons may present with confusion and coma followed by septic shock, disseminated intravascular coagulation and bleeding, acute respiratory distress syndrome, pericarditis, mild hepatitis, and multiorgan failure.
Other manifestations of F. tularensis infection include ulceroglandular tularaemia (75% to 85% of naturally occurring cases), acquired from an infected carcass or an arthropod bite. Symptoms are relatively mild and include fever, painful ulcerative skin lesions, and enlarged lymph nodes.
Glandular tularemia is similar but does not produce skin ulcers. The oculoglandular tularemia form of disease follows airborne exposure or exposure while cleaning contaminated animal carcasses. This form leads to ulceration of the cornea. Oropharyngeal tularemia is acquired by consumption of contaminated water or food, by hand-mouth contact, or by inhalation of infectious droplets. It is characterized by exudative pharyngitis or tonsillitis in the presence or absence of mucosal ulcers. Typhoidal tularemia is an acute flulike illness accompanied by diarrhea, vomiting, headache, chills, muscle and joint pain, prostration, and weight loss. This form of the disease follows ingestion or inhalation of bacteria. Without proper treatment, the mortality rate for type A tularemia is 5% to 15% overall, 4% for ulceroglandular tularemia, and 30% to 50% for typhoidal, septicemic, and respiratory forms. Use of antibiotics lowers the mortality rate to 1%. Type B tularemia is rarely fatal.
F. tularensis was first discovered to be a disease agent during a plaguelike outbreak of rodents in 1911. Its potential to cause human epidemics was seen during the 1930s and 1940s when large waterborne outbreaks occurred in Europe and the Soviet Union. It was one of the agents studied at the Japanese germ warfare research unit in Manchuria from 1932 to 1945 and was developed as a bioweapon by the Soviet Union and the United States. The former head of the Soviet bioweapons development effort, Kanadjan Alibekov, suggested that tularemia outbreaks affecting tens of thousands of Soviet and German soldiers during World War II may have been be due to intentional release. Military strains of F. tularensis were engineered for resistance to antibiotics and vaccines.
F. tularensis is a dangerous potential biological weapon due to its extreme infectivity (requiring fewer than ten inhaled bacteria to initiate pulmonary infection), ease of dissemination, and capacity to cause illness and death. Disease is, however, expected to progress more slowly and have a lower fatality rate than inhalational plague or anthrax. The lack of a stable spore phase complicates the bioterrorism application. If a weapon of airborne bacteria were released in a dense population center, however, it would be predicted to result in a large outbreak of pulmonary tularemia within three to five days. An aerosol dispersal of 50 kilograms of F. tularensis over a metropolitan area of 5 million inhabitants is projected to result in 250,000 incapacitated persons and 19,000 deaths. Illness may persist for weeks, with relapses potentially occurring for months. Vaccinated individuals may be only partially protected against such an exposure. Affected individuals should be promptly treated with streptomycin, gentamycin, doxycycline, or ciprofloxacin, which may also be useful as prophylactic agents. The Soviet Union immunized tens of millions of persons living in tularemia-endemic areas with a live attenuated vaccine. The United States also uses such a vaccine to protect laboratory workers routinely exposed to this bacterium. It is not known, however, if these vaccines provide protection against weaponized strains of F. tularemia.
Brucellosis and Brucella Species
Brucellosis (also known as Mediterranean fever, Gibraltar fever, Malta fever, Cyprus fever, undulant fever, and typhomalarial fever) is an ancient disease that remains the most commonly occurring zoonotic infection in the world. It is endemic to the Middle East, Central Asia, India, the Mediterranean regions of Europe and Africa, Central and South America, and Mexico. It is caused by infection with Brucella species, several small, highly contagious, gram-negative aerobic coccobacilli. Brucellosis is primarily a zoonotic infection that causes abortions in sheep and goats (B. melitensis), cows and bison (B. abortus), and pigs (B. suis). B. canis is pathogenic to dogs. Each of these bacterial species may induce human disease as well, although B. melitensis is the most pathogenic species and the one that most commonly infects humans.
Although Brucella species do not produce spores and are heat-sensitive, they are able to survive in the environment for several years under the correct conditions and continue to infect both humans and animals of the region during that period. Natural infection occurs primarily through consumption of unpasteurized milk and soft cheese or through areas of abraded skin (especially in slaughterhouse workers). Bacteria may also be acquired via mucous membranes, such as the conjunctiva lining the eyelids, the throat, and the respiratory tract—the route favored by bioterrorists. After entering a person, the bacteria may grow either outside of or within cells, such as macrophages and neutrophils, occupying special acidic compartments where they work to ensure the host cell’s continued survival. An unusual lipopolysaccharide in the cell wall inhibits killing of the bacteria by these phagocytic cells. Brucella also dampens the production of the cytokine TNF-α by other immune cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree