The discovery of the BRCA1 and BRCA2 mutation identifies a group of nearly homogeneous patients at substantially increased risk of breast cancer. The risk of breast cancer by age 70 in BRCA1 mutation carriers is estimated to range from 44% to 78% and for BRCA2 mutation carriers from 31% to 56%.1,2 Bilateral prophylactic mastectomy (BPM) is thus an important consideration for this population. Alternative approaches that have been investigated include frequent surveillance that includes the use of breast magnetic resonance imaging (MRI) and chemoprevention using tamoxifen.
Meijers-Heijboer and colleagues compared breast cancer related outcomes among BRCA1/2 mutation carriers who opted for BPM and compared this with those who chose to undergo surveillance.3 Many of the patients who underwent surveillance also had regular MRI of the breast. Among the 76 women who underwent BPM, no cases of breast cancer were identified after 2.9 years of follow-up. In contrast, 8 of 63 women who opted for a surveillance approach were diagnosed with breast cancer during this follow-up period. Importantly, among these 8 women, 1 death from breast cancer was also reported. Thus the authors estimated that the 5-year risk of breast cancer in the surveillance group was 12% with BPM reducing this risk by 66% to 100% (Fig. 65-1). In addition, although more women who underwent BPM also underwent prophylactic oophorectomy, the benefits of BPM in reducing breast cancer events was still significant even after controlling for this difference. Although data from this cohort suggested substantial benefit to BPM, the number of patients in the study as well as the follow-up was small, with a resulting wide estimate of risk reduction conferred by BPM. Thus this study leaves open the possibility that BPM in BRCA1/2 mutation carriers may confer less of an advantage than estimated by these initial findings.
Figure 65-1

Actuarial incidence of breast cancer among women with a BRCA1 or BRCA2. Mutation after prophylactic mastectomy or during surveillance. The surveillance group includes data obtained before prophylactic mastectomy in 76 of the 139 women. The dashed line represents the probability of breast cancer during surveillance, and the dotted lines represent the 95% confidence interval. Values were calculated with the use of an exponential model in which the hazard rate was assumed to be constant. [Reproduced, with permission, from Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345(3):159-164.]
Breast cancer related outcomes in a larger population of BRCA1/2 mutation carriers was reported by the PROSE study group.4 In this multicenter study, 483 women with deleterious BRCA1 or BRCA2 mutations were followed for a mean of 6.4 years. Of the 105 women who elected to undergo BPM, 2 cases of breast cancer (1.9%) were reported compared with 184 of 378 (48.7%) age-matched controls who did not undergo BPM. BPM thus afforded a 90% relative reduction in breast cancer risk, and this relative risk (RR) reduction increased to 95% when adjusted for prophylactic oophorectomy. More importantly, the absolute risk reduction seen with BPM was 46.8%. However, despite this dramatic difference, the PROSE study group did not provide survival outcomes between the 2 groups. Because women who undergo prophylactic mastectomy have a small but real risk of breast cancer as seen in the PROSE study and women in surveillance programs generally present with early-stage, highly curable cancer,5 it is possible that the breast cancer specific survival between the 2 groups would be less dramatic than the differences in breast cancer incidence.
Given the lack of survival data, Schrag and colleagues used a decision analysis model to predict the effects of prophylactic surgery on life expectancy among women with BRCA1 and BRCA2 mutations.6 They demonstrate substantial gains in life expectancy with prophylactic surgery, with the benefit maximized when intervention is carried out at earlier ages. Thus they estimated that a 30-year-old patient with a BRCA1 or BRCA2 mutation stands to gain 2.9 to 5.3 years of life with BPM (Fig. 65-2). Additional gains in life expectancy are also seen with the use of prophylactic oophorectomy, with little loss of this benefit if oophorectomy was delayed to the age of 40. These gains in life expectancy are on par with and in some cases exceed the benefits seen with other prophylactic medical interventions such as smoking cessation.
Figure 65-2
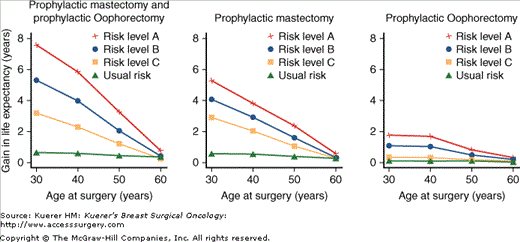
Gains in life expectancy among women with BRCA1 or BRCA2 mutations, according to age, cumulative risk of cancer, and type of prophylactic surgery. The graph shows the gains expected for 30-year-old women treated according to 3 prophylactic-surgery strategies, as compared with a strategy of no prophylactic surgery, according to age at the time of surgery for each of 4 levels of risk: the usual risk in the general population, risk level A (40% risk of breast cancer and 5% risk of ovarian cancer by the age of 70 years), risk level B (60% risk of breast cancer and 20% risk of ovarian cancer), and risk level C (85% risk of breast cancer and 40% risk of ovarian cancer). [Reproduced, with permission, from Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336(20):1465-1471.]
Data regarding efficacy of chemoprevention in the BRCA1/2 population are limited to subgroup analyses from large multicenter trials. Analysis of 288 breast cancer cases in the NSABP-P1 demonstrated that 8 patients were BRCA1 and 11 patients were BRCA2 mutation carriers.7 Of the 8 women with BRCA1 mutations who developed breast cancer, 5 had been randomized to tamoxifen and 3 to placebo (RR = 1.67; 95% confidence interval [CI], 0.032 to 10.7). Of the 11 women with BRCA2 mutations who developed breast cancer, 3 had received tamoxifen and 8 received placebo (RR = 0.38; 95%CI, 0.06 to 1.56). Although the risk reduction achieved with tamoxifen in the BRCA2 population was similar to that of the non-BRCA population, this reduction in breast cancer events in the BRCA2 cohort was not statistically significant given the small sample size. As noted in this study and several others, patients with BRCA1 mutations tend to predominantly develop estrogen receptor (ER)-negative tumors; thus there would be little expectation of achieving meaningful risk reduction among BRCA1 mutation carriers. However, the proportion of ER-positive cancers is higher in BRCA2 mutation carriers, and thus theoretically this population would derive benefit from antiestrogens as a prevention strategy. Nonetheless given the limited data, tamoxifen for chemoprevention is not widely used as a risk-reducing strategy among BRCA1/2 mutation carriers.
With development of an index cancer, women with BRCA mutations have substantial risk of a second contralateral breast cancer event. However, the role of routine contralateral mastectomy in this setting has not been established, and the data available in the literature are conflicting.
Metcalfe and colleagues followed 491 BRCA1/2 patients with stage I and stage II breast cancer and examined the effects of surgical and medical interventions on the risk of contralateral breast cancer. They found that women who do not undergo oophorectomy or receive antiestrogen therapy, have a 43.4% (BRCA1) and 34.6% (BRCA2) actuarial risk of a contralateral breast cancer 10 years following an initial cancer diagnosis.8 These risks were reduced following oophorectomy or tamoxifen therapy to 18.8% in BRCA1 patients and 13.1% in BRCA2 patients. However, the greatest reduction in contralateral breast cancer events was seen among women who underwent contralateral prophylactic mastectomy (CPM). Only 1 cancer (0.7%) was seen at an average of 9.2 years of follow-up among the 146 women who had CPM compared with 97 (28.8%) contralateral breast cancer cases among the 336 women who did not undergo CPM. Although these data show striking outcomes with CPM, a number of important limitations to the study are also noted. Most important among these was the lack of ER data on a significant number of the patient population, and similarly the relative distribution of ER status between CPM and no CPM groups was not known. In addition, a survival analysis to see whether the increased risk of death from index cancer was offset by the potential survival benefit afforded by preventing a contralateral breast cancer was not evaluated.
Survival end points were the focus of another report of CPM among BRCA1/2 mutation carriers.9 One hundred and forty-eight women with a history of stage I to IIIa breast cancer were followed for a mean of 3.5 years. Among this group, 79 women underwent CPM. The use of CPM also strongly correlated with the use of bilateral prophylactic oophorectomy (BPO). Although patients undergoing CPM had substantial reduction in contralateral breast cancer risk, after adjusting for BPO, no overall survival benefit was seen for those who had CPM. Additional analyses in this cohort demonstrated that the survival benefit was associated with BPO, rather than CPM, thus suggesting that the greater risk to survival among BRCA1/2 mutation carriers is from ovarian cancer rather than breast cancer. It is not clear whether with longer follow-up CPM would also be associated with improved survival.
Lastly, decision analysis using Markov modeling has also been applied to this setting to determine benefits in life expectancy from a number of different prophylactic interventions, including CPM, tamoxifen, and BPO.10 Maximal benefit in life expectancy was seen among women with high penetrance mutations, with early-stage breast cancer diagnosed early in life. The maximum gains in life expectancy were seen for a 30-year-old with early-stage breast cancer and ranged from 1.3 years for tamoxifen therapy to 2.1 years for CPM. These gains in life expectancy are substantially less than those anticipated for a 30-year-old woman who is a BRCA1/2 carrier without a breast cancer diagnosis who undergoes BPM. These differences speak to the need to additionally factor in prognosis from the index carcinoma when counseling BRCA1/2 mutation carriers who have already developed breast cancer. Thus the complex interplay between age, stage at diagnosis, and its subsequent impact on survival as well as mutation penetrance and probability of a contralateral breast cancer event all need to be carefully considered when counseling BRCA1/2 mutation carriers regarding CPM.
Women who are not BRCA1/2 mutation carriers yet are felt to be at increased risk due to family history or a history of lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH) at biopsy represent a very heterogeneous population of patients who may be considered for prophylactic mastectomy. Assessing future risk of cancer in this population has proven to be especially problematic, and differing definitions have been used when examining the effect of prophylactic mastectomy in this population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







