Syndrome
Rate of thyroid cancer
Associated conditions
Pattern of inheritance and mutations
Gardner’s syndrome (FAP)
2–12 % with PTC [15] with mean age at diagnosis of 28 years
Gastrointestinal polyps
Osteomas
Epidermoid cysts
Desmoid tumors [16]
Autosomal dominant tumor suppressor APC gene
Cowden’s syndrome (PTEN hamartoma syndrome)
Two-thirds with thyroid pathology including multinodular goiter, follicular adenomas, FTC, and PTC [17]
Multiple hamartomas
Breast cancer
Endometrial cancer
Autosomal dominant tumor suppressor PTEN gene
Carney complex
15 % of patients with PTC and FTC [18]
Adrenal and pituitary gland pathology
Myxomas of the soft tissue, heart, skin, and brain
Schwannomas
Testicular tumors
Lentigines
Autosomal dominant
PRKA1α gene
Werner syndrome
18 % of patients with thyroid malignancy including PTC, FTC, ATC [19]
Soft tissue sarcomas
Melanomas
Osteosarcomas
Autosomal recessive
WRN gene
Familial Non-medullary Thyroid cancer
3.2–9.4 % of all thyroid cancer cases [20]
Differentiated thyroid cancer of follicular origin in two or more first-degree relatives [21]
Not identified
MEN 2, FMTC
MEN 2A – pheochromocytoma, hyperparathyroidism
MEN 2B – pheochromocytoma, mucosal neuromas, ganglioneuromatosis of GI tract, megacolon
Autosomal dominant
RET proto-oncogene
Symptoms
Most patients with thyroid nodules have no hyper- or hypothyroid symptoms. They are often asymptomatic; a minority of nodules can be detected by physical examination, and more are detected incidentally with imaging. If the nodules are palpable, patients can present with a slowly enlarging mass in the neck. The most common symptoms associated with thyroid nodules are those due to pressure on or invasion of adjacent structures and can include dysphagia, globus sensation, compressive/constrictive feeling in the neck, hoarseness or change in speech quality, difficulty breathing, and cosmetic concerns. Occasionally, they can also cause anterior neck pain with radiation to the ears [7]. There can be pain in the thyroid itself when there has been acute hemorrhage into the nodule and there is associated inflammation or rapid growth [24]. In extremely large goiters, patients can have a Pemberton’s sign, which is facial flushing upon elevation of the arms above the head, due to partial obstruction of the superior vena cava [7]. If a nodule is visible and palpable, it is possible to deduce how quickly the nodule has grown and if symptoms have been stable or changing. Patients who present with symptoms of tracheal or esophageal compression, vocal cord paralysis, or persistent hoarseness may be more likely to harbor malignancy [14].
If nodules are functioning, they can be associated with symptoms of hyperthyroidism. Autonomously hyperfunctional thyroid nodules can be either solitary or multiple and can lead to thyrotoxicosis. If a thyroid nodule is noted in a patient with symptoms of tachycardia, anxiety, tremor, heat intolerance, weight loss, and frequent bowel movements, this is suggestive of a toxic thyroid nodule. However, Graves’ disease can also present with hyperthyroid symptoms [25] along with a diffusely enlarged thyroid, or asymmetric enlargement, which can simulate a nodule. Additional clinical characteristics that can be associated with Graves’ disease, that are not seen with a toxic nodule, include the presence of ophthalmopathy and dermopathy. Patients with ophthalmopathy, or thyroid eye disease, can have periorbital edema, proptosis, exophthalmos, lid lag, stare, and conjunctival injection [26]. Dermopathy can present as non-pitting edema, usually of the lower extremities in the pretibial area. If a patient presents with thyroid dysfunction associated with ophthalmopathy, the pretibial area should be carefully examined for subtle signs of dermopathy, such as localized thickening of the skin with reddish discoloration [27].
A slowly growing, diffusely enlarged thyroid or thyroid nodule associated with typical symptoms [28] such as dry skin, cold sensitivity, fatigue, muscle cramps, voice changes, and constipation could suggest hypothyroidism.
Family History and Cancer Syndromes
Several familial syndromes are associated with increased risk of thyroid cancer. These include Gardner’s syndrome, PTEN hamartoma syndrome (Cowden’s syndrome), Carney complex, Werner syndrome, familial non-medullary thyroid carcinoma, multiple endocrine neoplasia type 2 (MEN 2), and familial medullary thyroid carcinoma (FMTC) [15–23, 29] (Table 2.1).
Hirschsprung disease and McCune-Albright, Peutz-Jeghers, Pendred, and ataxia-telangiectasia syndromes also have been reported to be associated with thyroid cancer, but the links are less established [29]. If family history is significant for any of these syndromes, it is important to not only evaluate for thyroid nodules but also for the non-thyroid manifestations of these syndromes (Table 2.1).
Radiation Exposure
Studies have shown that children exposed to ≥1 Gy of ionizing radiation are at higher risk of development of thyroid nodules at a rate of 2 % annually [30], and these nodules have a higher risk of malignancy, estimated at 20–50 % [31]. This risk can persist for over 50 years [31]. Additionally, a history of external radiation exposure in low or medium doses (40–50 Gy) given to patients with lymphoma or head and neck cancer, particularly in childhood, is a highly concerning risk factor for both benign and malignant nodules [32].
Iodine Exposure
The risk of thyroid disease from iodine exposure is U-shaped which shows potential harm for the patients from both iodine deficiency and iodine excess [33]. Iodine deficiency and excess can both cause thyroid dysfunction; iodine deficiency has also been associated with a diffusely enlarged thyroid and goiter [34] and can trigger formation of nodules. The possible mechanism is due to chronic stimulation by TSH and the effects of increased reactive oxygen species in the iodine-deficient thyroid [35].
Physical Examination
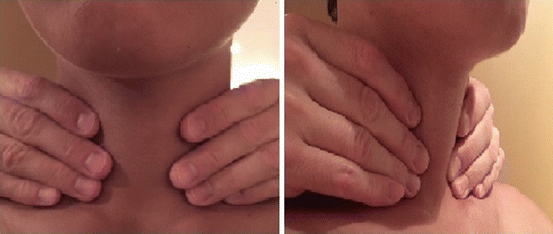
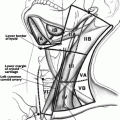
Knowing how to perform a good physical examination of the thyroid and neck is essential. First, it is important to know the anatomy of the neck, which will help the examiner identify essential landmarks (Fig. 2.1). The thyroid is made up of a right and left thyroid lobe and the connection in the middle is the isthmus. Some people may have a pyramidal lobe, which extends superiorly from the isthmus (usually on the left), just lateral to the midline [7]. Each lobe is approximately 2–4 cm in size and the right lobe can be slightly larger than the left. When palpating the thyroid gland, one should first palpate the cricoid cartilage. The isthmus is usually situated caudal to it; hence, identifying the cricoid cartilage first will indicate where the isthmus should be [7]. Once the isthmus is located, then the right and left lobes can be identified and palpated medial to the sternocleidomastoid muscles. Examination should include both inspection and palpation of the neck in good light with the patient sitting upright with their neck straight or slightly extended [36]. In this position, goiters and many nodules can be visible when swallowing. For some patients, it may be necessary to provide a cup of water to facilitate swallowing [36].
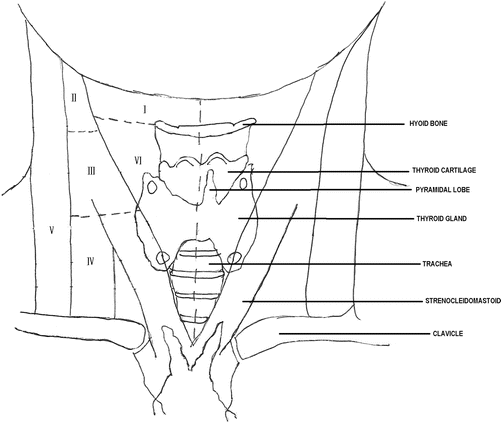

Fig. 2.1
Neck anatomy including cervical lymph node levels
The neck and thyroid can be examined using two different methods. The first is with the examiner sitting or standing behind the patient and using both hands to come around anteriorly to palpate the thyroid gland (Fig. 2.2). Both lobes and the isthmus are palpated simultaneously with the pads of the second, third, and fourth fingers. The second method is for the examiner to stand to the side of a seated patient and examine the thyroid and neck with one hand, using the pads of the thumb for one lobe and the pads of the second, third, and fourth fingers for the opposite lobe (Fig. 2.3). The isthmus can be palpated in the same fashion. After palpation of the thyroid, examination of the neck lymph nodes, level 1–6 (Fig. 2.1), should be performed, and this is usually done with the examiner either behind the patient or facing the patient and using both hands to palpate both sides of the lateral neck.