The Cancer and Leukemia Group B (CALGB) was founded in 1956 and is now “a national network including 26 university medical centers, over 225 participating community hospitals and more than 3,000 oncology specialists who collaborate in clinical research studies aimed at reducing the morbidity and mortality from cancer, relating the biological characteristics of cancer to clinical outcomes and developing new strategies for the early detection and prevention of cancer.”1
CALGB investigators have designed and conducted a number of pivotal clinical trials addressing treatment of breast cancer. CALGB is a member of the Breast Cancer Intergroup of North America where it regularly leads and participates in Intergroup trials. This chapter highlights trials led by the CALGB.
CALGB trials have addressed systemic therapy questions in breast cancer patients including optimum total dose of drug, schedule of drug delivery and dose density, combination versus sequential administration of different agents, as well as assessment of different agents. Many CALGB trials incorporate a factorial design in which patients are randomized into 2 or more arms for the first portion of treatment and then undergo a second randomization to 2 or more different arms for the second part of treatment. This approach allows testing of multiple hypotheses within the same trial.
CALGB has consistently had a strong correlative science program with companion laboratory studies that collect tissue, blood, and data from patients on trial to address mechanism of response, predictors of response, and other questions. For example, a correlative science study assessed different methods for assessment of HER-2 overexpression, and demonstrated fluorescence in situ hybridization (FISH) was a reliable method for measuring HER-2 expression.
A series of CALGB trials addressed questions of total dose of chemotherapy, dose density of chemotherapy delivery, and administration of agents sequentially versus in combination for early stage breast cancer.
By 1985, the NIH Consensus Conference concluded that the use of chemotherapy in node-positive premenopausal women was standard of care, while the efficacy of chemotherapy in node-positive postmenopausal women was unclear, although some studies suggested a benefit.2 Building on this basic belief, the CALGB worked to further clarify the role of adjuvant chemotherapy.
CALGB 8541: Dose and Dose Intensity of Adjuvant Chemotherapy for Stage II, Node-Positive Breast Carcinoma
CALGB 8541 addressed 2 main questions regarding CAF chemotherapy in both pre- and postmenopausal women with node-positive breast cancer. The impact of dose of drug administered per cycle was assessed using 3 different doses. The impact of dose density versus duration of treatment was also addressed as 2 of the arms administered the same total amount of drug, one over 6 cycles and the other in only 4 cycles with a higher dose per cycle.
Patients were randomized to 1 of 3 regimens of cyclophosphamide, doxorubicin, and fluorouracil (CAF):
- Group 1 (high dose) 600/60/600 mg/m2 IV every 28 days for 4 cycles
- Group 2 (moderate dose) 400/40/400 mg/m2 IV every 28 days for 6 cycles
- Group 3 (low dose) 300/30/300 mg/m2 IV every 28 days for 4 cycles
The trial enrolled 1572 patients, making it one of the largest prospective trials performed at that time. After a median follow-up of 3.4 years, Wood et al reported that increasing the dose of doxorubicin from 30 to 60 mg/m2 improved disease-free and overall survival with acceptable toxicity.3
This study was significant because it was one of the first to show a difference in survival resulting from the use of different doses of chemotherapy over varying time intervals. It helped define the concept of a threshold effect for breast cancer chemotherapy (ie, that doses below a critical level show significantly reduced efficacy). Whereas both the moderate- and high-dose regimens delivered the same cumulative dose, there was a trend favoring the high-intensity regimen. This observation would lead to a later trial (CALGB 9741) directly assessing the impact of dose density.
CALGB 9344/Intergroup 0148: Adjuvant High-Dose versus Standard Dose AC with and Without Paclitaxel in Node-Positive Breast Cancer
The novel mechanism of action of paclitaxel, its lack of cross-resistance with anthracyclines, and its manageable toxicity led to interest in incorporating it into adjuvant chemotherapy regimens. This led to several studies of its use in the treatment of early breast cancer.
CALGB 9344/INT0148 enrolled 3121 patients with node-positive breast cancer between 1994 and 1999 into a 3 × 2 factorial design trial comparing different doses of doxorubicin plus cyclophosphamide with or without the addition of paclitaxel. Patients were randomized to a control arm of the most effective doxorubicin dose from CALGB 8541 (60 mg/m2), versus even higher doses (75 or 90 mg/m2) administered with cyclophosphamide (600 mg/m2) for 4 cycles. Patients then underwent a second randomization to an additional 4 cycles of paclitaxel (175 mg/m2) versus observation (Fig. 50-1). This trial design allowed comparison of the impact of the use of paclitaxel or not on 3 different doses of doxorubicin with cyclophosphamide.
Figure 50-1
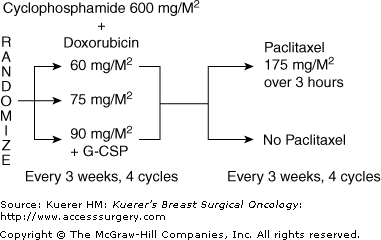
CALGB 9344 protocol schema; 3 × 2 factorial design. After completion of chemotherapy, radiation therapy was administered if patient was treated with lumpectomy or at discretion of physician if patient was treated with mastectomy, and tamoxifen 20 mg/d was administered for 5 years if the tumor was receptor positive. (Reproduced, with permission, from Henderson IC. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976-983.)
After completion of chemotherapy, radiation therapy to the breast was administered if the patient was treated with lumpectomy or at the discretion of the investigator if the patient was treated with mastectomy (Fig. 50-2). Tamoxifen (20 mg PO daily) was administered for 5 years if the tumor was hormone-receptor positive.
Figure 50-2
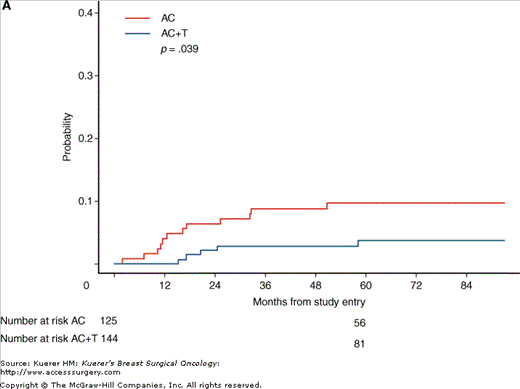
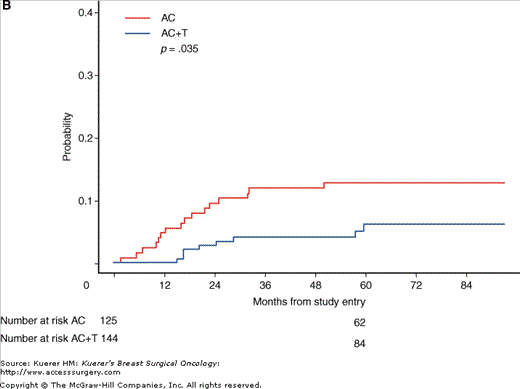
CALGB 9344. Breast-conserving therapy with radiotherapy. A. Cumulative incidence of isolated locoregional recurrence; B. cumulative incidence of locoregional recurrence as any component of failure. AC, doxorubicin plus cyclophosphamide; AC+T, AC plus paclitaxel. (Reproduced, with permission, from Sartor CI, et al. Effect of addition of adjuvant paclitaxel on radiotherapy delivery and locoregional control of node-positive breast cancer: Cancer and Leukemia Group B 9344. J Clin Oncol. 2005;23:30-40.)
The addition of paclitaxel was shown to improve both disease-free and overall survival.4 At 5 years, 70% of women who received paclitaxel were disease-free, compared with 65% of women who did not. Overall survival was 80% in the paclitaxel group versus 77% in the no-paclitaxel group. There was no benefit seen with doses of doxorubicin above 60 mg/m2. A subset analysis based on estrogen receptor status showed significant benefit from the addition of paclitaxel to those women whose tumors lacked estrogen receptors, although this correlation of benefit with estrogen receptor status was not confirmed by a similar NSABP trial.5
This trial also demonstrated that the increased time between surgery and radiotherapy resulting from delivery of additional cycles of paclitaxel after doxorubicin and cyclophosphamide did not adversely impact local control.6 In fact, the addition of paclitaxel afforded better local control than doxorubicin and cyclophosphamide alone in patients treated with breast-conserving therapy.
CALGB 9741/Intergroup C9741: A Randomized Trial of Dose Dense versus Conventionally Scheduled and Sequential versus Concurrent Combination Chemotherapy as Postoperative Adjuvant Treatment of Node-Positive Primary Breast Cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







