1. Nondiagnostic or unsatisfactory
2. Benign
3. Atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS)
4. Follicular neoplasm/suspicious for follicular neoplasm (including oncocytic lesions)
5. Suspicious for malignancy
6. Malignant
In the years following the publication of this reporting system, multiple studies confirmed the utility of the Bethesda terminology [17–21]. Few institutional modifications and suggestions were also reported [22–28]. However, currently, this system seems to be the most widely accepted terminology for reporting thyroid cytology in the literature. It is important, however, to note that institutional variations depending on patient populations and interobserver variability among cytopathologists are well established [29–32]. Therefore, it is highly recommended that each practice collect their own data with case distributions and malignancy risks. We will look into the categories in detail and point out possible areas of weakness, particularly in the “indeterminate” categories, with clinical implications and malignancy risks for each category.
Components of Thyroid Fine Needle Aspiration
Follicular Cells
The main cellular components of thyroid FNA are the follicular cells (see Fig. 5.1), the primary functional cells of thyroid parenchyma, responsible for production of thyroid hormones. Cells are arranged in three-dimensional groups of variable sizes with colloid in the central lumen storing thyroglobulin. On FNA specimens, cells may be seen individually or forming intact follicles on smeared slides. C-cells, responsible for calcitonin production, are not identified in thyroid FNAs unless they form a neoplastic mass.
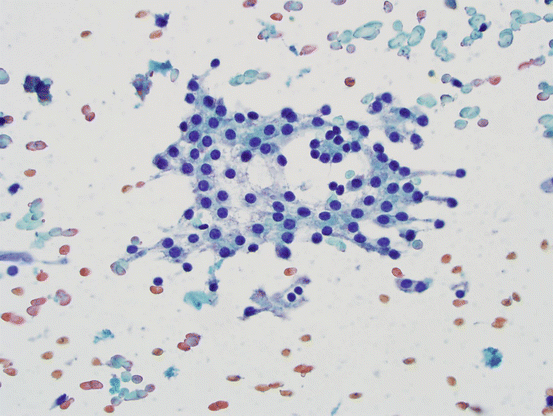

Fig. 5.1
Follicular cells. Bland follicular cells with round-to-oval nuclei, smooth nuclear contours; cytoplasm is fine and friable
Oncocytic (Hürthle) Cells
These are large, epithelial cells with abundant, granular cytoplasm, engorged with mitochondria (see Fig. 5.2). Although the term oncocyte (meaning “swollen cell”) may be more appropriate, Hürthle cell terminology has been entrenched in medical practice and is used commonly. While similar morphologic changes can also be seen in thyroid C-cells, the term Hürthle cell implies a follicular cell origin.
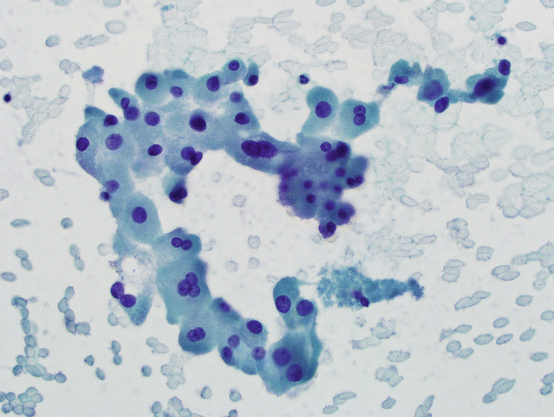

Fig. 5.2
Hürthle cells. Group of cells with abundant, granular cytoplasm and single or multiple, round-to-oval, smooth nuclei. There may be prominent nucleoli
Colloid
Colloid is the storage form of thyroglobulin that is packed inside follicles in the thyroid. It is a homogeneous, viscous material with characteristic smearing pattern on FNA slides. Pathologists who perform thyroid FNAs can easily identify colloid grossly by the smearing characteristics and shiny, smooth, and homogeneous, honey-like features on glass slides before fixation.
Inflammatory Cells
Both acute and chronic inflammatory cells can be seen in thyroid FNAs and may be secondary to infectious and autoimmune inflammatory processes and neoplasias of the hematolymphoid system.
Macrophages
Macrophages serve as scavenger cells in tissue. In the thyroid, they may be seen in association with thyroid cysts, where they are characterized by their vacuolated (“foamy”) cytoplasm with or without pigment (mostly hemosiderin) (see Fig. 5.3).
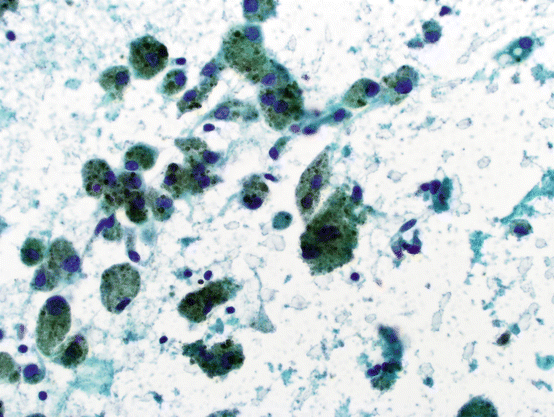

Fig. 5.3
Cyst contents with abundant pigment-laden histiocytes
Stromal and Vascular Components
Depending on the underlying pathologic processes, vascular, stromal, neural, or skeletal muscle fragments may be present in thyroid FNA specimens.
Diagnostic Categories
Nondiagnostic or Unsatisfactory
The main reason for specifying adequacy of thyroid FNA specimens is to avoid false-negative diagnoses. To reduce the risk of false negatives, the cytopathologist should be able to identify the tissue appropriately. This, however, involves multiple parameters including the operator, slide preparation, proper fixation and staining, and also the inherent characteristics of the nodule itself, such as solid vs. cystic components, hemorrhage into the lesion, degenerative or necrotic changes and amount of sclerosis, calcification, or ossification involving the nodule. Therefore, there is no single criterion for the adequacy of thyroid FNAs. It should also be noted that the adequacy discussion applies to specimens that would otherwise be reported as benign; in other words, if a specimen is considered for any diagnosis other than benign, it should not be reported as nondiagnostic or unsatisfactory, but instead findings should be communicated in an appropriate manner in the pathology report. In this context, the value of detailed verbal or written communication cannot be overemphasized. While in certain practices, a terminology of nondiagnostic implies the features and findings are not “diagnostic for a specific entity,” and the term unsatisfactory is used when there is insufficient material for proper evaluation; the two forms a single diagnostic category in the Bethesda system.
One of the earlier reports on quantitative criteria for adequacy was from Dr. Goellner at Mayo Clinic giving actual numbers of follicular cells necessary for adequacy [33]. While “adequacy” reflects much more than the number of follicular cells on glass slides, this proposal by Goellner has remained useful for reporting thyroid cytology for decades and was also included in the Bethesda terminology. For this purpose, six groups of well-visualized cells, each with ten follicular cells, should be considered an “adequate” specimen for evaluation of thyroid nodules in the appropriate setting. This means that the cytologic specimen should be sufficient to identify the “lesion,” with clinical and preferably radiologic correlates.
The exceptions to the quantitative requirements for adequacy are those that would identify the lesion in the thyroid as anything other than benign or otherwise guide the clinical or surgical management of the patient. Examples include colloid nodules or inflammatory processes where the follicular cell component may not be well represented or not present at all in the aspirate smears.
Cyst contents without sufficient follicular epithelial cells are considered nondiagnostic. The main concern for these cases is a cystic papillary thyroid carcinoma. In such instances, the aspirates are reported as nondiagnostic with a statement that the FNA shows “cyst contents only.” Still, such smears have a very low risk of malignancy particularly for nodules smaller than 4 cm in size and those that shrink after the FNA procedure [34].
Similarly, obscuring blood, preservation and/or fixation artifacts, and staining problems can render the specimen nondiagnostic even if the cellularity is quantitatively “sufficient.”
While there are wide variations in the literature for the nondiagnostic category, overall it averages around 10 % [14, 15, 35–40]. In a meta-analysis including a large series in the post-Bethesda era, Bongiovanni reported an average nondiagnostic rate of 13 %, ranging from 1.8 to 23.6 %, in over 25 thousand FNAs [20].
The risk of malignancy for nondiagnostic specimens is difficult to assess in small series without sufficient follow-up, because the majority of these cases do not lead to surgical intervention. The studies that report a malignancy risk for this group with surgical follow-up overestimate the malignancy risk because of selection bias, i.e., the patients with surgical follow-up usually have additional indications for excision, such as increasing size, clinical symptoms, or abnormal or suspicious findings on imaging that skew the risk stratification for these patients. Overall, the malignancy risk with nondiagnostic specimens is actually very low. While it ranges from 0.6 to 39 % in different series, depending on how the data is collected, the malignancy risk is especially low for nodules without suspicious radiologic findings and smaller lesions [15, 35–37, 39, 41, 42]. In a study including 393 cases with an original nondiagnostic FNA but with adequate cytologic, surgical, or ultrasound follow-up, only 2.3 % were associated with malignancy [41]. In this series, the risk increased significantly with each 1 cm increase in any dimension of the nodule [41].
The overall inadequacy rate may decrease with ultrasound guidance [2, 3]. On-site evaluation of thyroid FNAs, with or without USG, may also prove helpful in further reducing the nondiagnostic rate of thyroid FNAs [38, 43, 44]. However, it should be emphasized that more important than any USG or aspiration technique is the experience and competency of the operator performing the procedure and also the cytologist evaluating the specimen [45]. The Bethesda system recommendation for nondiagnostic aspirates is a repeat FNA but “no sooner than 3 months later,” preferably with ultrasound guidance and rapid, on-site adequacy evaluation. While ultrasound guidance is likely to reduce the nondiagnostic rate, similar to on-site evaluation, there is no convincing data in the literature that requires a specific time interval for a repeat aspirate. Actually, recent studies that looked into this recommendation did not find any basis for a 3-month period in their series [46, 47]. Furthermore, no contraindication is proven for immediate repeat aspirate, either. On the other hand, it seems reasonable to allow the tissue repair to prevent overinterpretation of reparative/reactive changes as an atypical or neoplastic process particularly by an inexperienced cytopathologist. However, additional factors, including patient compliance, clinical and ultrasonographic findings, and operator experience should all be considered in deciding the most appropriate follow-up. This is particularly evidenced by studies that showed clinical and radiologic follow-up was as acceptable as a repeat aspirate for initially nondiagnostic thyroid FNAs, particularly in the absence of suspicious radiologic findings [41, 48, 49].
Benign
While FNA diagnosis of thyroid nodules can be utilized for confirmation of malignancy or determination of the extent of surgery, the primary purpose of a thyroid FNA is to document that the nodule is benign and no surgical excision is necessary. As the overwhelming majority of thyroid nodules are benign, in most practices, at least 60 % of thyroid FNAs are reported as such [9, 11, 14, 15, 33]. Therefore, thyroid FNA has been an extremely useful tool in prevention of many unnecessary thyroidectomies. When a benign diagnosis is rendered on cytology, the nodule can safely be followed clinically and radiologically, and no further immediate diagnostic studies are indicated [50].
The benign diagnosis includes multiple entities, including benign follicular nodule, colloid nodule, and inflammatory conditions.
Benign follicular nodule is the most common diagnosis for thyroid FNAs. As the name implies, this group consists of follicular-patterned lesions, which encompasses a large and diverse group of lesions including the broad term of “follicular hyperplastic nodules” and also some “follicular adenomas.” Follicular hyperplastic nodules include multinodular or uninodular goiters, dominant hyperplastic nodules, nodules in the background of Graves’ disease, and colloid nodules (see below). Generally, differentiation of these entities on cytology has little or no clinical significance, as their clinical management will be the same, or in the case of Graves’ disease, the diagnosis is usually established on clinical grounds.
The main cytologic characteristic of a benign follicular nodule is presence of colloid and a mixture of bland follicular cells, commonly including Hürthle cells. Therefore, proper identification of colloid on cytologic material is very important. It is common for colloid to “wash off” with fixation. Therefore, it is best seen on stained air-dried smears as dark blue-magenta-colored material. Colloid may be thick, dark, and cracked, or it may be “watery” as clouds of bluish tinge on smears (see Fig. 5.4). To an untrained eye, it may be difficult to differentiate colloid from serum.

Fig. 5.4
Colloid. Homogeneous, viscous, gel-like material that forms smooth smears on glass slides. It may crack or fold on the edges
When specimens show abundant colloid, even in the absence of follicular cells, those cases are reported as “benign” or “colloid nodule” as the malignancy risk for such lesions is considered to be extremely low [51]. However, in practice, this is a relatively rare occurrence. These can be considered as one end of the spectrum of “macrofollicular lesions.” The term colloid nodule should be reserved for those lesions that are clearly dominated by definite colloid on smears. Additionally, the cytologic findings should be supported by the imaging characteristics of the nodule sampled. A specimen with abundant colloid should not be reported as benign or adequate if the ultrasonographic features are consistent with a solid lesion.
In addition to colloid, follicular epithelial cells are commonly seen in smears from benign follicular nodules (see Fig. 5.5). They may be seen as sheets or follicles of various sizes. It is important to note that a minor component of microfollicles can be seen in benign follicular nodules, and presence of microfollicles in such a background should not be interpreted as atypical or follicular neoplasm. Follicles show a range of sizes and three-dimensional intact follicles can be seen. As the size of the follicles decrease, it is more likely to see colloid in the center of the follicles. Depending on the aspiration technique and the size of the needle, occasional thick tissue fragments may be seen; however, in a fine needle aspiration specimen, three-dimensional groups of follicles (instead of occasional individual follicles) should not be seen. Cellularity may be low to moderate and occasionally marked; however, there is a mixture of follicular architecture, ranging from small to large macrofollicles, flat sheets, and occasional microfollicles.
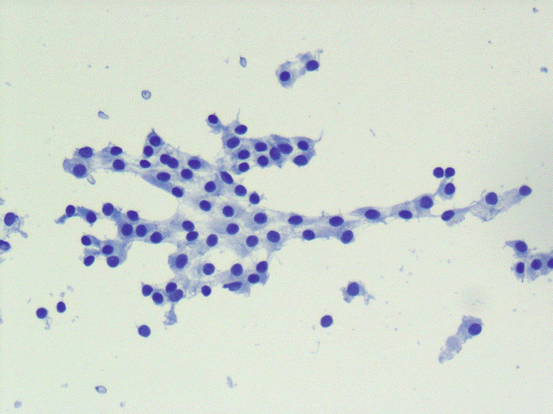

Fig. 5.5
Benign follicular nodule. Bland follicular epithelial cells without nuclear atypia
It is at this point pertinent to mention what constitutes a microfollicle. So far, the best definition of a microfollicle is by Renshaw as “less than 15 cells, arranged in a circle that is at least two-thirds complete, and flat.” Microfollicles can also be seen as small, compact, three-dimensional “spheres” with colloid in the center.
Follicular epithelial cells are bland, with moderate to abundant cytoplasm. Cytoplasm may be smooth or granular depending on the amount of cytoplasmic organelles and the metabolic activity of the cells. During the aspiration and smearing of the specimen, the cytoplasm may be ripped off, and scattered naked nuclei may be present in the background. Nuclei of normal follicular cells have a very slight variation in size, shape, and chromatin pattern. They are round to oval, monotonous cells with smooth, homogeneous chromatin. The nuclear membrane is usually very smooth and regular, without indentations, grooves, or intranuclear inclusions. Occasionally, one or two small, inconspicuous nucleoli may be seen but without angulations.
Hürthle cells or oncocytes are commonly seen as a part of benign follicular nodules. While Hürthle cell terminology for oncocytic lesions of the thyroid is a misnomer as Hürthle cells are actually the C-cells of dogs [52], it has been well established in the literature and clinical practice to use the name Hürthle for oncocytic cells in this location. Hürthle cells have abundant cytoplasm filled with mitochondria, which gives a homogeneously granular appearance to these cells. Nuclei are round to oval, moderately enlarged, and usually with a single prominent nucleolus. Hürthle cells may also show marked nuclear enlargement, membrane irregularities, and hyperchromasia, which should not be interpreted as atypia or malignancy. It should be noted that there may be “early Hürthle cells” with features intermediate between bland follicular epithelial cells and Hürthle cells.
Background is usually dominated by colloid and may be bloody. Bloody background is a sign of high vascularity and more commonly seen with neoplastic nodules; however, needle size, aspiration technique, and medications such as blood thinning agents may be related to markedly bloody aspirates.
The benign follicular nodule category should not be considered a diagnosis of exclusion. A nodule should not be diagnosed as benign if features are not diagnostic for any specific lesion. The cytopathologist should identify the features of a benign follicular nodule for appropriate diagnosis.
Thyroiditis
Hashimoto Thyroiditis
Lymphocytic thyroiditis and Hashimoto thyroiditis seem to be different phases of an autoimmune disease characterized by autoantibodies against thyroid-related antigens. Immune-mediated injury with both cellular and antibody-mediated mechanisms leads to tissue damage, regeneration, and eventually exhaustion of the tissue leading to the morphologic changes.
Four characteristic histo-morphological features of Hashimoto thyroiditis are:
- 1.
Lymphocytic infiltration with germinal centers
- 2.
Hürthle cell metaplasia
- 3.
Follicular atrophy (microfollicles)
- 4.
Fibrous bands of scarring (fibrous variant)
However, Hashimoto thyroiditis is not a single pathologic entity, and there is a wide variation of morphologic features differing in severity and predominant morphologic feature on histology (see Fig. 5.6). Similarly, features of Hashimoto thyroiditis on cytology show marked variations. The major cytologic features of Hashimoto thyroiditis (see Fig. 5.7) are:
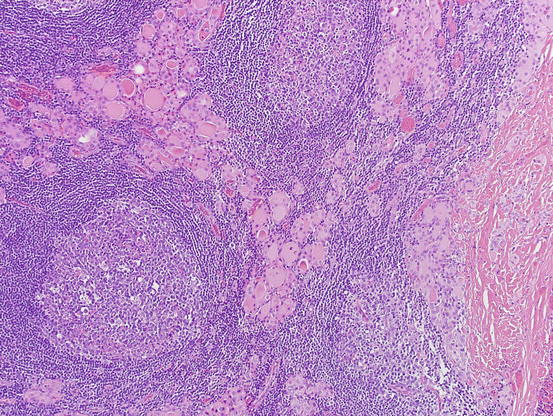
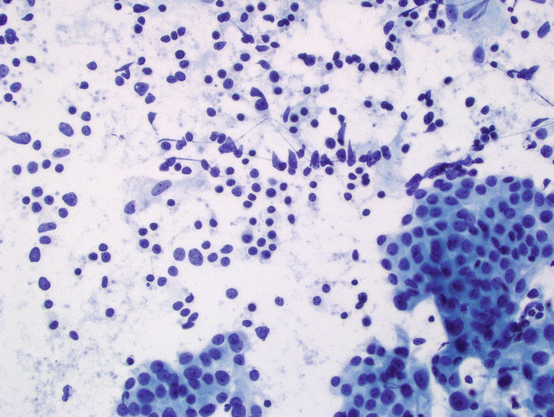

Fig. 5.6
Hashimoto thyroiditis. Histologic section shows thyroid parenchyma infiltrated by abundant lymphoid cells with germinal centers. Follicular epithelial component shows atrophy and prominent Hürthle cell changes

Fig. 5.7
Hashimoto thyroiditis. Most characteristic features of Hashimoto thyroiditis are cellular smears with variable amount of lymphocytic infiltrate and Hürthle cells
- 1.
Presence of a mixed population of lymphoid cells including small, mature lymphocytes, reactive lymphocytes, and occasional plasma cells. Germinal centers may be identified on cytologic smears.
- 2.
Sheets and scattered Hürthle cells with granular cytoplasm, enlarged nuclei, and prominent nucleoli usually predominate the follicular epithelial component on cytology.
- 3.
Presence of microfollicles is not a sign of follicular neoplasia and should not be over-interpreted in these cases.
- 4.
Stromal fragments with capillaries are also seen associated with Hashimoto thyroiditis.
Commonly, the lymphocytic cells seem to intermingle with the epithelial clusters; however, the only finding of Hashimoto thyroiditis may be a slight but definite chronic inflammatory infiltrate in the background of a cellular thyroid aspirate with a mixed Hürthle cell population.
No minimum cytologic requirements are established for diagnosis of Hashimoto thyroiditis on cytology. Some require identification of all four components, including capillaries in smears, while others may report presence of a lymphoid infiltrate as evidence of lymphocytic (Hashimoto) thyroiditis.
Granulomatous Thyroiditis
This is an idiopathic disease, usually seen in middle-aged women with painful thyroiditis, often with fever. It is usually bilateral; however, it may be asymmetrical and rarely aspirated. Diagnosis of granulomatous thyroiditis may be possible on aspiration cytology; however, the cellularity varies depending on the activity of the inflammation. The findings are those of a granulomatous inflammation with foreign body type, multinucleated giant cells, the most characteristic finding of this disease (see Fig. 5.8); however, it should be emphasized that multinucleated giant cells can commonly be seen in a variety of thyroid aspirates with and without malignancy, and mere presence of multinucleated cells should not be interpreted as granulomatous thyroiditis. Epithelioid histiocytes and well-formed granulomas may be seen on cytology, some surrounding colloid. Follicular epithelial cells may be abundant in the background, including Hürthle cells. Colloid may be minimal.
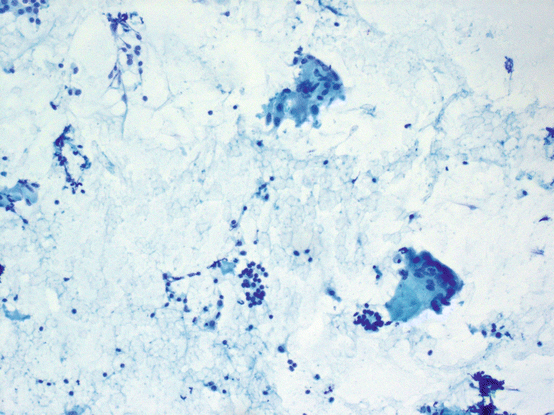

Fig. 5.8
Subacute thyroiditis. Bland follicular cells, lymphocytes, and multinucleated giant cells are seen
Acute Thyroiditis
Acute thyroiditis has a typical clinical presentation and usually is not subjected to FNA, unless a drainage and microbiology culture are planned. FNA material shows abundant acute inflammation and background debris (see Fig. 5.9). Follicular epithelial cells may be a minor component in the background if identified at all. Epithelial cells usually show reactive atypia, which should not be interpreted as neoplastic.
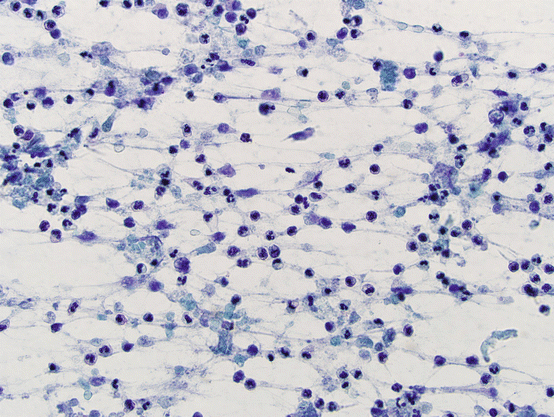

Fig. 5.9
Acute thyroiditis. Acute inflammatory infiltrate. Follicular cells may not be identified in smears
Graves’ Disease
Graves’ disease is an autoimmune thyroiditis, more commonly seen in middle-aged women as diffuse hyperplasia of the thyroid. The patients are usually diagnosed clinically with hyperthyroidism. The disease usually involves the thyroid in a diffuse fashion and is not aspirated. Occasionally, asymmetrical involvement and nodules may be seen on imaging or palpation that may be followed with FNA.
The cytologic features of Graves’ disease are not specific and clinical correlation is very helpful. Overall, findings are similar to other benign follicular nodules; however the cellularity may be marked and raise concern for follicular neoplasia. Smears are cellular with mixed follicular cells showing micro- and macrofollicles, a very helpful feature in differentiating these lesions from follicular neoplasms, particularly for those cases where the background colloid is minimal. Occasional papillary hyperplastic groups may be seen, but clinical history and absence of nuclear atypia should steer the cytologist from over-interpreting these as papillary carcinoma.
A mixed lymphoid background with or without Hürthle cells may be present; however, it is usually much less pronounced than Hashimoto thyroiditis.
Riedel Thyroiditis
This is a very rare form of thyroiditis characterized by marked fibrosis extending outside the thyroid parenchyma, raising concern for malignancy. Cytologically, specimens are usually not cellular and may show mixed chronic inflammation with relative lack of follicular cells and colloid. In this clinical setting, the most important finding is the absence of a cytologically diagnostic malignancy, such as anaplastic carcinoma or sarcoma.
Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance (AUS/FLUS)
This is the most controversial category in the Bethesda system; however, it serves a specific purpose in reporting and management of thyroid nodules with certain morphologic features. It should be noted that this category does not pertain to a single type of lesion or pathologic correlate but should rather be considered to be any type of lesion identified on cytology with a malignancy risk higher than benign but lower than follicular neoplasia/suspicious for follicular neoplasia or suspicious for malignancy, i.e., in the range of 5–15 % [16]. This is the range that is considered by some to be not high enough for immediate surgical intervention but too high for routine follow-up. The definition of AUS/FLUS is broad and covers multiple scenarios. The most common scenarios described in the Bethesda system involve those lesions where there is a small but definitive concern for a follicular neoplasm, Hürthle cell neoplasm, or malignancy (papillary thyroid carcinoma); however, atypia involving lymphoid or other cells types including medullary carcinoma can also be in this category. The lesion may not be well represented on the aspirate slides due to various reasons such as low cellularity, obscuring hemorrhage, and preservation/staining artifacts, or the clinical background may only partially explain the atypia seen in the specimen, such as Hashimoto thyroiditis, history of radiation exposure, or drugs such as carbimazole.
In the Bethesda system, specific situations are listed with a final “not otherwise categorized.” To prevent overutilization of this category as a “wastebasket” diagnosis, a recommendation was made to limit this category in the range of 7 % of all thyroid FNAs. As expected, the reproducibility of this category is far from perfect [32] and the terminology is used differently in many practices [23, 53]. Additionally, the recommended malignancy risks for this group showed marked variability in the literature, usually exceeding the expected range of 5–15 % [27, 54–61].
Since BSRTC became more widely accepted and used, the risk stratification of the atypical category showed significant variations not only among institutions but also how it is reported. While the Bethesda system does not recommend subdividing the AUS/FLUS category, many suggested that the atypical category should be reported in subgroups with descriptive qualifiers as they represent different risks of malignancy. Considering the recent reported findings in the current literature, this category can, at least theoretically, be divided into the following subgroups [27, 54–62]:
- 1.
AUS with nuclear atypia, concerning for papillary thyroid carcinoma (PTC); however, findings are not sufficient for a diagnosis of “suspicious for malignancy” or “malignancy.” These mostly include atypia in a limited number of cells. There is a significant body of literature showing that this group has the highest risk of malignancy, ranging from 28 to 56 %.
These cases show focal nuclear enlargement and nuclear membrane irregularities including grooves and homogenous pale chromatin in an otherwise benign FNA (see Fig. 5.10). It should be noted that, in many series, it may be appropriate to place these cases into the “suspicious for malignancy” category instead of AUS. It is of great importance for the cytopathologist to interpret cytologic atypia and features of PTC appropriately. Only those cases that the nuclear features cannot be explained by reactive changes in the background of Hashimoto thyroiditis, history of radiation, medications, identifiable cyst-lining cells, etc., should be reported as atypical. Similarly, if there is a pattern of atypical features or a separately identifiable population of cells with atypical features raising concern for PTC, those should be reported as “suspicious for malignancy.” In many institutions, any intranuclear cytoplasmic invaginations (INCI) seen in follicular epithelial cells on a well-fixed, appropriately stained slide are reported as at least suspicious for malignancy, not AUS. For these reasons, the reader should become familiar with the diagnostic features of PTC described in the sections below.
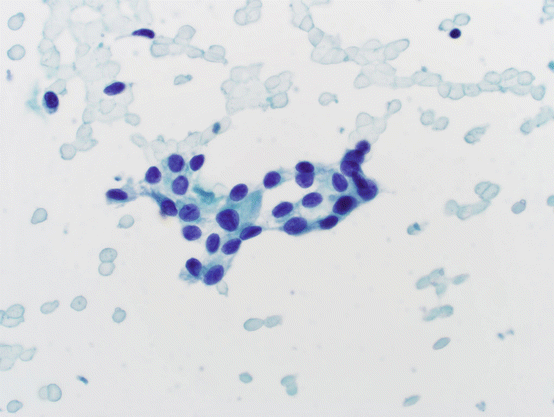
Fig. 5.10
Atypical of undetermined significance. Small, cohesive epithelial cells with finely granular chromatin and nuclear grooves. Nuclei are somewhat more elongated than round. No Hürthle cell morphology is evident. In isolation, this epithelial group raises concern; however, it is not sufficient for the cytologic diagnosis of suspicious for PTC or malignancy
AUS with prominent microfollicles in a sparsely cellular specimen or in the background of a mixed pattern where findings are not supportive of a diagnosis of follicular neoplasm (see Fig. 5.11). Overall, the risk of malignancy in this category is relatively low, on average 5–25 %, depending on how the data is obtained. In our experience, the risk is closer to the lower end of the spectrum.
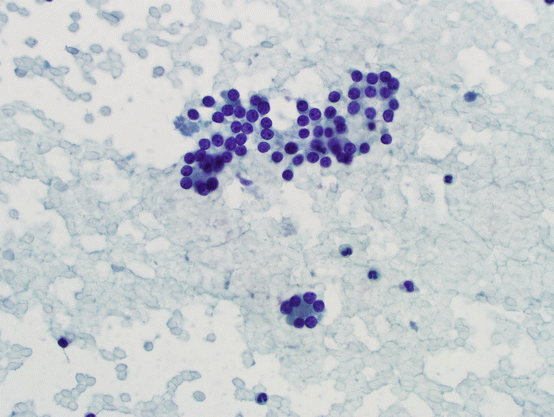
Fig. 5.11
Microfollicles. Bland follicular cells forming flat or three-dimensional groups with 15 cells or less
- 2.
AUS with predominance of Hürthle cells in a sparsely cellular specimen or in the background of Hashimoto thyroiditis or multinodular goiter. This category seems to be more heterogeneous and complex; however, it seems to have a very low risk of malignancy, less than 10 % in most series.
Some authors further characterize Hürthle cells as those with dysplasia and those without dysplasia; however the reproducibility of this practice is not well established, mostly because Hürthle cells associated with benign proliferations commonly show nuclear enlargement, chromatin clumping, hyperchromasia, nuclear membrane irregularities, and degenerative changes with or without high nuclear-to-cytoplasmic (N/C) ratios. However, if there is a monotonous population of Hürthle cells, particularly without background benign features or Hashimoto thyroiditis, it is still appropriate to report these cases AUS/FLUS.
- 3.
AUS, not otherwise specified (NOS), involving other cellular components, including lymphoid cells in Hashimoto thyroiditis or atypia that cannot be characterized due to specimen processing and staining problems. History of radiation exposure including radioactive iodine and other drugs may also show nuclear atypia, which may be diagnosed as AUS/FLUS. Cytologic changes seen in cyst-lining cells can also be in this group. Obviously, this is a mixed group with overall risk of malignancy averaging 8–36 %.
It is important to clearly communicate the cytologic findings in the pathology report. If the clinical team is not aware of the significance of the findings and relative risks that are associated with individual diagnoses, it is best to include a comment about clinical significance in reporting individual cases. This is best accomplished by obtaining institutional data as there is significant variation in risk of malignancy in different practice settings [55, 63].
In most practices, the next step after a diagnosis of AUS/FLUS is repeat aspiration. Similar to the discussion for nondiagnostic aspirates, an appropriate interval of 3 months has been suggested, but there is no evidence to support this interval. In over half of AUS/FLUS cases, a repeat FNA will be diagnostic, most often with a benign diagnosis [37, 64–66], thus significantly reducing unnecessary thyroid surgery.
It should also be noted that the malignancy risk associated with only surgically excised cases show an erroneously elevated malignancy risk for this category, as those cases may have additional clinical or imaging findings suspicious for malignancy.
Follicular Neoplasm/Suspicious for Follicular Neoplasm (Including Oncocytic Lesions)
Follicular-patterned lesions form the largest and most heterogeneous group in the thyroid ranging from benign, non-neoplastic follicular hyperplasias to follicle-forming infiltrating carcinomas. These lesions share common morphologic features on cytology, and FNA is not a reliable tool for differentiating these lesions on cytologic grounds. Diagnosis of malignancy relies on histologic evidence of an infiltrative lesion, which cannot be assessed on aspiration specimens. Therefore, a follicular neoplasia (FN) diagnosis on cytology covers the main differential of cellular hyperplastic nodules, follicular adenomas, follicular carcinomas, and follicular variant of papillary thyroid carcinoma (FVPTC). A recently updated terminology for some of the lesions that used to be included in the category of encapsulated follicular variant of papillary carcinoma, i.e., “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP), should also be included in this differential (see discussion below) [67]. In a recent study, 56 % of these benign lesions that were surgically removed had a preoperative cytologic diagnosis of follicular neoplasm [68]. In addition, some rare tumors, such as medullary carcinomas, poorly differentiated thyroid carcinomas, parathyroid proliferations, and some metastatic carcinomas, may also be reported as FN [69].
In the Bethesda system, the FN category is considered to carry a malignancy risk of 15–30 %. Considering the heterogeneous nature of this category, it is possible to divide this group into subgroups with separate morphologic characteristics and corresponding malignancy risks.
- 1.
Bland hypercellular follicular lesions: Benign follicular hyperplasia is the most common histologic lesion in the thyroid, and FNA can easily classify these lesions as benign with high sensitivity and specificity. However, those lesions with marked cellularity, overcrowding, and abundant microfollicles with scant background colloid enter the spectrum of FN (see Fig. 5.12). Even in histologic sections, definitive diagnosis depends on complete evaluation of the entire capsule of the lesion, which is not possible on cytology. Therefore, the spectrum of lesions in this group includes cellular hyperplastic nodules on one end and invasive follicular carcinomas on the other. Increased nuclear-to-cytoplasmic (N/C) ratios, marked cellularity, dyscohesion, and three-dimensional clusters have been suggested as possible signs of “malignancy” in this group; however, these have not been proven to be reproducible in larger studies [70, 71].
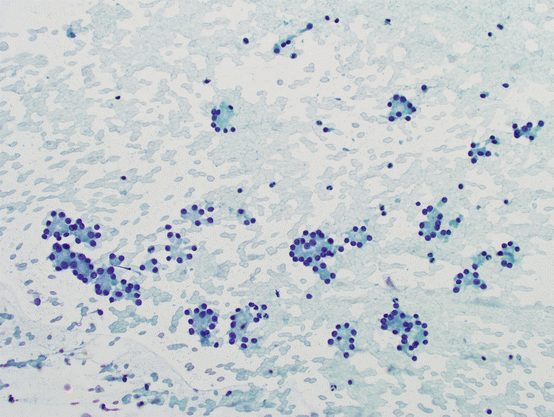
Fig. 5.12
Bland hypercellular follicular lesions. Bland follicular epithelial cells with abundant microfollicles in the background of scant to absent colloid. No nuclear atypia is present
The border between the Bethesda system categories of AUS/FLUS and FN is not well defined; however, increased cellularity with microfollicles and “uniformity” of both cells and architecture are reliable signs of neoplasia. Characteristically, there are more cells than colloid in FN.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree