The Austrian Breast and Colorectal Cancer Study Group (ABCSG), formerly known as “Cooperative Studiengruppe Mammakarzinom,” was established in 1984 as a cooperative institution conducting multicenter clinical trials in breast and colorectal cancer. Since its inception, more than 20,000 patients have been enrolled in some 30 prospective randomized phase III studies. The key scientific treatment issues studied until now in major clinical programs have been (1) adjuvant endocrine therapy for pre- and postmenopausal women, as well as the role of bisphosphonates, and (2) chemotherapy in the neoadjuvant setting. The goal of this chapter is to provide a brief synopsis of the high-impact trials carried out by the ABCSG with respect to breast surgical oncology and to examine their study results in a broader context.
ABCSG Trial 5 (ABCSG-5) and Trial 12 (ABCSG-12) were clinical investigations exploring the efficacy of adjuvant endocrine treatment in premenopausal patients with early-stage breast cancer.
When ABCSG-5 was initiated in 1990, the adjuvant treatment of premenopausal breast cancer patients was considered to be a domain of adjuvant chemotherapy. Yet the fact that premenopausal women responded significantly better to adjuvant chemotherapy than older patients raised the hypothesis that this effect could mainly be mediated through endocrine manipulation rather than being a direct effect of cytostatic action.1 Supported by initial reports on 2 other modalities tamoxifen (TAM) and ovarian ablation2,3—ABCSG-5 was designed to compare the efficacy of combination endocrine treatment with standard chemotherapy.
ABCSG-5 randomized patients to receive either 3 years of the luteinizing hormone-releasing hormone analog goserelin (GOS) plus 5 years of TAM or 6 cycles of cyclophosphamide, methotrexate, and fluorouracil (CMF; Table 47-1). The trial subjects were stratified according to their hormone receptor status, tumor stage and grading, number of involved nodes, and type of surgery.
| Criteria | ABCSG-5 | ABCSG-12 (BMD Substudy) |
|---|---|---|
| Reference | Jakesz et al4 (2002) | Gnant et al9 (2008) |
| Regimen | TAM 20 mg/d, 5a + GOS 3.6 mg every 28 days, 3a vs CMF 600/40/600 × 6 every 4 weeks | TAM 20 mg/d, 3a + GOS 3.6 mg every 28 days, 3a ± ZA 4 mg every 6 months, 3a vs ANA 1 mg/d, 3a + GOS 3.6 mg every 28 days, 3a ± ZA 4 mg every 6 months, 3a |
| No. of assessable patients | 1034 | 404 |
| Characteristics | pT1–3, N±, G1–3,x | T1a–T4d, N±, G1–3,x |
| Primary end points | OS, RFS | Change in BMD |
| Recruitment period | December 1990 to June 1999 | June 1999 to May 2006 |
| Median follow-up (months) | 60 | 48 |
Results of the final analysis were published in 2002.4 A total of 1034 assessable patients had completed a 60-month median follow-up period. By that time, 17.2% of patients in the endocrine treatment group and 20.8% of those undergoing chemotherapy had developed relapses. The data showed a significant difference in relapse-free survival (RFS; 81% vs 76%, p = 0.037; Fig. 47-1) and local RFS (95% vs 92%, p = 0.015) in favor of endocrine therapy. This translated into a 40% increase in the relative risk of experiencing a relapse for patients treated with CMF as compared with those receiving TAM plus GOS (relative risk = 1.4, 95% confidence interval [CI] 1.06–1.87). In summary, ABCSG-5 provided evidence that complete endocrine blockade with GOS and TAM is superior to standard chemotherapy in premenopausal women with hormone-responsive stage I and II breast cancer.
Figure 47-1
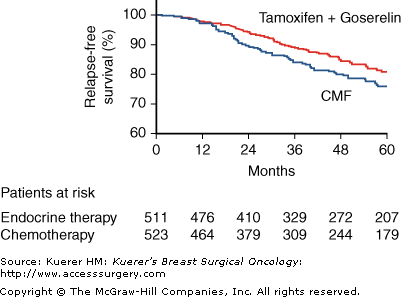
Kaplan-Meier estimates of relapse-free survival in the group assigned to endocrine therapy (tamoxifen and goserelin) and the group assigned to CMF. Differences were significant (p = 0.037, log-rank test). (Reprinted with permission from Jakesz R, Hausmaninger H, Kubista E, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20:4621-4627.)
The data generated with ABCSG-5 correlate well with those from other studies showing that adjuvant endocrine therapy with GOS, either alone or in combination with TAM, is at least as effective as CMF-based chemotherapy in this setting with regard to overall survival (OS) and disease-free survival (DFS).5,6 These trials finally resulted in a recommendation to use adjuvant endocrine therapy as an alternative to chemotherapy in premenopausal women with hormone-responsive breast cancer in the framework of the consensus guidelines developed at the 2001 International Conference on the Adjuvant Therapy of Primary Breast Cancer in St Gallen.7
The rationale for TAM as standard of care for more than 20 years is based on evidence that it reduces the risk of recurrence and improves survival in patients treated for 5 years.2 However, it is also associated with an increased risk of endometrial cancer and vascular events. Deduced from the data generated with third-generation aromatase inhibitors (AIs) in the adjuvant postmenopausal setting, either as a replacement therapy for or as follow-up to TAM, ABCSG Trial 12 was the first study to investigate the combination of GOS with an AI in premenopausal women. Its objective was to assess the benefit of adjuvant treatment with the combination of GOS plus either TAM or anastrozole (ANA) on DFS and OS in premenopausal patients with hormone-responsive cancer. Due to the observed significant loss of bone mineral density (BMD) associated with AI therapy, a prospectively defined BMD subprotocol was initiated to evaluate the effect of concomitant zoledronic acid (ZA) on BMD.
ABCSG-12 was launched in 1999 and finally enrolled a total of 1803 women. Following ovarian suppression with GOS, patients were randomly assigned to 1 of 4 treatment groups and received either TAM ± ZA or ANA ± ZA together with GOS as basic therapy (Table 47-1). Within the bone substudy protocol, BMD was measured at 0, 6, 12, 36, and 60 months in 404 women.
The first outcome results of ABCSG-12 were presented in the Highlights Plenary Session of the 2008 Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago.8 The full publication appeared in the New England Journal of Medicine in 20099, and data showed no significant difference in DFS between ANA and TAM. However, the addition of ZA to endocrine therapy, yielded a reduction of 36% in the risk of disease progression (hazard ratio [HR] = 0.64, 95% CI 0.46-0.91, p = 0.01) at 4 years of follow-up.9 Mature data deriving from this clinical program thus demonstrate that ZA added to adjuvant endocrine therapy improves outcome in premenopausal patients with endocrine-responsive disease.
In addition, in its first published analysis (at 36 months’ follow-up), the substudy assessing ZA for preventing BMD loss already showed a significant degree of loss in patients who received endocrine therapy alone (BMD: 17.3% vs 11.6%, p < 0.001) and maintenance in BMD in women who received endocrine therapy plus ZA (BMD -14.4%, p = 0.001) as compared to those not given ZA.10 In the final analysis 2 years after completion of treatment (median follow-up: 48 months), patients not receiving ZA still had decreased BMD (p = 0.001, 2-sample t-test), while those receiving ZA had increased BMD (p = 0.02, 2-sample t-test) as compared to baseline (Figure 47-2).11
Figure 47-2
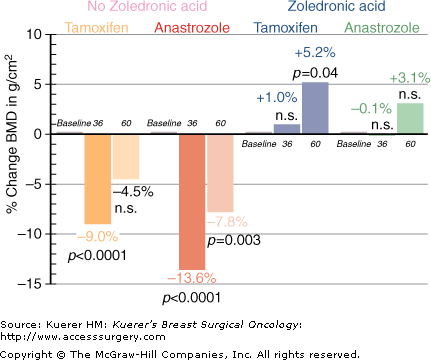
Percentage change in lumbar spine bone-mineral density from baseline to 36 and 60 months. Patients were randomly assigned to anastrozole or tamoxifen with or without zoledronic acid for 36 months and then no treatment from 36 to 60 months. (Reprinted with permission from Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679-691.)
In conclusion, ABCSG-12 showed that significant BMD loss caused by endocrine therapy for premenopausal women with breast cancer can be effectively inhibited by the administration of ZA. Moreover, the survival data provide evidence that the antitumor activity of adjuvant ZA improves the outcome of premenopausal breast cancer patients beyond the effect of endocrine therapy.
The objective of the ABCSG-6, ABCSG-6a, and ABCSG-8 (± Arimidex-Nolvadex [ARNO] 95) trials was to evaluate the effect of combined adjuvant endocrine therapy (TAM plus AI) given simultaneously or sequentially for the treatment of endocrine-responsive breast cancer in postmenopausal patients.
Adjuvant endocrine therapy after primary surgery for breast cancer has been shown to reduce the risk of recurrence and increase OS beyond the period of treatment for women with estrogen receptor–positive disease when ABCSG-6 was conducted.12 Today, the substantial and persistent benefit from adjuvant TAM compared with no adjuvant treatment has been well demonstrated.13 The intention driving ABCSG-6 was to answer the question whether the results of adjuvant TAM could be improved by the addition of other endocrine agents in an attempt to target TAM-resistant cell clones or perhaps to suppress the partially agonist action shown by TAM.14 Aminoglutethimide (AG), one of the first AIs that became available for clinical use and shown to be effective in the treatment of postmenopausal women with advanced breast cancer, was used in this study. The follow-up ABCSG-6a trial was an extension of ABCSG-6 that aimed to investigate the efficacy of extended adjuvant therapy with ANA in patients who remained recurrence-free after 5 years of adjuvant TAM.
ABCSG-6 was launched in 1990 and assigned a total of 1986 assessable trial participants to receive either TAM alone for 5 years or TAM in combination with AG for the first 2 years of treatment. ABCSG-6a included 852 patients who had received 5 years of adjuvant TAM with or without AG and who were free of disease by the end of ABCSG-6. These trial participants were re-randomized to receive either 3 years of ANA or no further therapy (Table 47-2).
| Criteria | ABCSG-6 | ABCSG-6a | ABCSG-8 (± ARNO 95) |
|---|---|---|---|
| Reference | Schmid et al15 (2003) | Jakesz et al16 (2007) | Jakesz et al21 (2005) |
| Regimen | TAM 20 mg/d, 5a* + AG 500 mg/d, 2a† vs TAM 20 mg/d, 5a* | ANA 1 mg/d, 3a vs 0 | TAM 20 mg/d, 2a‡ → ANA 1 mg/d, 3a vs TAM 20 mg/d, 5a‡ |
| No. of assessable patients | 1986 | 852 | 3224 |
| Characteristics | pT1b–pT3a, N±, G1–3,x | pT1–pT3a, N±, G1–3,x | T1–3, N±, G1–3,x |
| Primary end points | OS, RFS | RFS | EFS |
| Recruitment period | December 1990 to December 1995 | March 1996 to March 2001 | January 1996 to June 2004 |
| Median follow-up (months) | 63.6 | 62.3 | 28 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







