The American College of Surgeons Oncology Group (ACOSOG) was established primarily to evaluate the surgical management of patients with malignant solid tumors. The ACOSOG includes general and specialty surgeons, representatives of related oncologic disciplines, and allied health professionals in academic medical centers and community practices throughout the United States of America and abroad.
The ACOSOG is 1 of 10 cooperative groups funded by the National Cancer Institute (NCI) to develop and coordinate multi-institutional clinical trials and is the only cooperative group whose primary focus is the surgical management of patients with malignant solid tumors. The Cooperative Group Program was established in 1955 with an initial Congressional appropriation of $5 million. Continued growth has led to a progressive increase in funding, with an NCI appropriation of $154 million for the Cooperative Group Program in 2001. The plan to develop a new oncology cooperative group focused on surgical therapies originated in 1993. The American College of Surgeons (ACS) Board of Regents approved the concept and established a working committee of 7 surgical oncologists to devise a plan for the group’s organization and structure. The committee’s work was supported through a planning grant from the NCI and from funds appropriated by the ACS Board of Regents. Many individuals contributed to the effort, including surgical oncologists, radiation and medical oncologists, and biostatisticians.
After developing a set of clinical trials for a broad range of surgical specialties, a grant was submitted to the NCI, and a site visit was held at Washington University in St. Louis in June 1997. Upon the recommendation of the NCI subcommittee H, the grant was funded with an official start date of May 15, 1998. The ACOSOG was initially based at the American College of Surgeons administrative office in Chicago, Illinois, and was led by the group chair, Dr Samuel A. Wells. In January 2001, the ACOSOG moved its operations to the Duke University Medical Center. This move allowed the ACOSOG to form an association with the Duke Cancer Center and with the Statistics and Data Center based at Duke University. In addition, the ACOSOG formed an alliance with the Duke Clinical Research Institute, a well-established academic clinical research organization. After the ACOSOG grant was refunded in December 2004, Dr Wells passed the reigns to Drs Heidi Nelson and David Ota who currently share the Group Chair position. Dr David Ota, MD, FACS, oversees the ACOSOG Administrative Coordinating Center (ACC) at Duke University in Durham, North Carolina. Dr Heidi Nelson, MD, FACS, provides scientific leadership for the group and is based at the Mayo Clinic Cancer Center in Rochester, Minnesota. Dr Nelson recruited Dr Karla Ballman from the Mayo Clinic Cancer Center in Rochester, Minnesota, to lead the ACOSOG Statistics and Data Center (SDC). The ACOSOG SDC is currently based at the Mayo Clinic Cancer Center, Rochester, Minnesota, under the direction of Dr Karla Ballman. The Central Specimen Bank, funded by U24-CA114736-03 from the NIH, houses all of the biospecimens collected in the conduct of ACOSOG trials and is based at Washington University, St. Louis, Missouri, under the direction of Dr Mark Watson.
The ACOSOG is dedicated to developing clinical trials for patients with a cancer diagnosis and to conducting trials that are relevant to surgeons and the surgical management of solid tumors. The ACOSOG is strongly committed to educating surgeons in the regulations and conduct of clinical trials and to providing leadership in the cooperative group setting. The ACOSOG originally had a very broad portfolio with trials opened for patients with brain tumors, breast cancer, gastrointestinal malignancies, head and neck tumors, lung cancer, melanoma, prostate cancer, and soft tissue sarcomas. These trials were conducted by several disease site committees and working groups. More recently, the ACOSOG has sharpened its focus to four scientific committees (Basic Science, Breast, Gastrointestinal, and Thoracic). The Basic Science Committee, originally chaired by Dr Joseph Nevins of Duke University, has a major emphasis on genomics and proteomics. The committee leadership has recently been transitioned to Dr Elaine Mardis at Washington University in St. Louis, Missouri. The remaining scientific committees focus on the diseases with the greatest cancer burden in the United States: the Breast Committee, chaired by Dr Kelly K. Hunt from the MD Anderson Cancer Center in Houston, Texas; the Gastrointestinal Committee, co-chaired by Dr Mitchell C. Posner from the University of Chicago in Chicago, Illinois, and Dr Peter W. T. Pisters from the MD Anderson Cancer Center; and the Thoracic Committee, chaired by Dr Joe B. Putnam from Vanderbilt University in Nashville, Tennessee. Through this committee activity, ACOSOG has enrolled over 18,000 patients on phase II and III clinical trials and demonstrated that surgeons can conduct and complete multicenter trials within several disease sites. The activities of ACOSOG are also supported through the work of administrative committees including Audit, Constitution and Bylaws, Data Monitoring, Diagnostic Imaging, Education, Ethics, Membership, Nursing/CRA, Patient Advocates, Radiation Oncology, and Special Populations. A Peer-Review and Prioritization Committee was developed to ensure broad-based scientific review of concepts and protocols from each of the disease site committees prior to submission to the Cancer Therapy and Evaluation Program (CTEP) of the NCI.
The ACOSOG has renewed its NCI funding and is a cooperative group led by surgeons with strong multidisciplinary participation with the goal of improving the care of the surgical oncology patient through an innovative clinical research program. The scientific themes of the ACOSOG are
To test novel therapies that may increase response rates and cure rates and reduce morbidities and disabilities associated with cancer care
To conduct basic science studies in conjunction with clinical trials to better understand the biologic basis of diseases and treatments
To support individual members and investigator networks to accrue patients to trials and fulfill the scientific mission of the ACOSOG
The membership criteria of the ACOSOG are designed to encourage broad participation by general surgeons and subspecialty surgeons, as well as other physicians and allied health professionals in academic medical centers, community hospitals, and private practice settings. Patient registration on ACOSOG studies can only be performed by physicians. The membership model of ACOSOG was originally designed to be individual investigator–based in order to increase participation from community surgeons. The membership quickly burgeoned to over 4100 members with 2167 surgeons, 1040 allied health professionals, 370 medical oncologists, and 273 radiation oncologists. The ACOSOG continues to support individual membership but is now building investigator networks within each of the disease sites (breast, gastrointestinal, and thoracic). These investigator networks are intended to facilitate the conduct of clinical trials and increase patient accrual while allowing the ACOSOG to fulfill its scientific mission. The trials run by the ACOSOG are relevant to both academic and community oriented surgeons and currently over 60% of enrollment on ACOSOG trials comes from surgeons in community practices who have incorporated clinical research into their daily practice. The ACOSOG leadership has endeavored to build on past successes, and to match the interests of the member networks in developing the clinical trials portfolio of the group. Membership interest based on accrual has indicated that the evolving scientific agenda should be focused on patients with malignancies of the breast, thoracic cavity, and gastrointestinal system.
The ACOSOG Breast Committee chair, Dr Kelly Hunt, is supported by 3 vice chairs, Dr Rache Simmons of the Weill Cornell Medical College, Dr Pat Whitworth of the Nashville Breast Center, and Dr Marilyn Leitch of the University of Texas Southwestern Medical Center. The Breast Committee has 3 aims, which are embodied within the research goals of the ACOSOG. The first is to improve cure rates and individualize care of patients through neoadjuvant therapies and molecular studies. The second is to increase prognostic accuracy for individual patients through investigations of novel biomarkers for risk stratification. And third, to improve the lives of our breast cancer patients through less invasive or less aggressive local-regional therapies, while not compromising the local-regional control that is so important in the long-term outcomes.
ACOSOG Z0010—a Prognostic Study of Sentinel Node and Bone Marrow Micrometastases in Women with Clinical T1 or T2 N0 M0 Breast Cancer
The Z0010 trial was designed to evaluate the incidence and impact of sentinel node and bone marrow micrometastases on patients with early-stage carcinoma of the breast treated with breast-conserving surgery and radiation therapy. The overall objective is to determine whether or not positivity in each compartment represents a different biological pathway and a different degree of prognostic significance. The study chair for Z0010 is Armando Giuliano, MD, of the John Wayne Cancer Institute in Santa Monica, California. This trial was activated in April 1999 with an accrual goal of 5300 patients. The trial was completed in May 2003 with a final accrual of 5539 patients. The ACOSOG was the only participating group in this trial.
The primary objective of ACOSOG Z0010 was to estimate the prevalence and evaluate the prognostic significance of sentinel node micrometastases detected by immunohistochemistry (IHC), and to estimate the prevalence and evaluate the prognostic significance of bone marrow micrometastasis detected by immunocytochemistry (ICC). The secondary objectives were to evaluate the hazard rate for regional recurrence in women whose sentinel nodes are negative by hematoxylin and eosin (H&E) staining and to provide a mechanism for identifying women whose sentinel nodes contained metastases detected by H&E so that these women could be considered as candidates for ACOSOG Study Z0011.
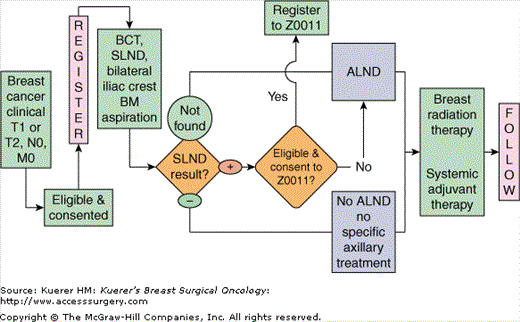
ACOSOG Z0010 was a phase II trial where all patients enrolled were planned for breast-conserving therapy (BCT) and sentinel lymph node dissection (SLND) (Fig. 44-1). Participants in the trial underwent bilateral anterior iliac crest bone marrow aspiration biopsies followed by segmental mastectomy and SLND. Bilateral bone marrow aspirates and sentinel lymph nodes found to be negative by H&E were submitted for ICC and IHC to the central lab. The results of the sentinel node and bone marrow micrometastasis studies were blinded to the individual investigators and decisions regarding the use of systemic therapy were left to the discretion of the treating clinician based on primary tumor factors. If a sentinel lymph node could not be identified during the SLND, a level I and II axillary lymph node dissection (ALND) was performed. Patients with negative sentinel lymph nodes, based on standard H&E sections, did not receive any specific axillary treatment (ALND or radiation). Patients with metastasis identified in the sentinel node(s) on H&E sections were eligible for registration and randomization on ACOSOG Z0011. Patients with a positive sentinel node who did not participate in Z0011 were treated with ALND. All patients received whole-breast radiation therapy.
Patients were followed at 6-month intervals for 5 years and then annually thereafter and assessed for local and regional recurrence, contralateral breast primary tumors, distant recurrence, and death. Special assessments for surgical side effects in the axilla and ipsilateral upper extremity were performed on all patients.
Eligible patients were female patients with clinical stage I or II (T1 or T2 N0 M0) invasive breast cancer amenable to treatment with breast-conserving surgery. Patients with prior ipsilateral axillary surgery, prepectoral breast implants, bilateral breast cancer, multicentric disease, or prior chemotherapy or hormonal therapy for the index breast cancer were excluded. Pregnant and lactating patients were also excluded from study participation.
Bone marrow aspiration was initially an optional procedure; however, this was later changed to a mandatory procedure for all study participants.
In order to enroll patients in this study, surgeons were required to have documented experience in performing SLND. Surgeons were allowed to participate in the Z0010 study after submitting documents demonstrating their experience in performing SLND with complete axillary lymph node dissection (ALND), by verifying training in the SLND technique through a surgical residency or fellowship training program, or through participation in an institution-wide validation study of SLND. Dr Lisa Newman, chair of the Special Populations Committee, obtained funding and initiated a project to educate surgeons working in underserved communities in the technology of sentinel node surgery who did not have training or access for this procedure to offer to their patients.
From May 10, 1999 through May 30, 2003, 5539 patients were enrolled on Z0010 from 112 physician groups. Patient data eligibility review was conducted on the study population and revealed the ineligibility rate to be approximately 3%.
Surgeons from 126 different institutions participated in Z0010 with 48% academic, 20% teaching affiliated, and 29% community practice. Almost 75% of the patients were enrolled by 28% of the participating surgeons. Twenty-four percent of surgeons accrued 75% of minority patients. Female surgeons accrued 24% of patients and accounted for 30% of the study investigators. A survey of the participating surgeons revealed that 16% of respondents reported no prior experience with clinical trials.1
The median age of patients enrolled in Z0010 was 56 (range 23-95, see Table 44-1). A total of 3602 bone marrow specimens and 3729 sentinel node specimens were obtained and sent to the central lab for analysis. Immunocytochemistry (ICC) and immunohistochemistry (IHC) analyses of the bone marrow and lymph nodes, respectively, were performed on submission of the specimens to the central lab. Dr Richard Cote and his colleagues at the University of Southern California completed a review of all bone marrow and sentinel node specimens. In addition, a second external review of all positive bone marrow cases was completed by pathologists at the NCI.
| Age | ||
| Median | 56 | |
| Minimum | 23 | |
| Maximum | 95 | |
| Race | ||
| White | 4808 | 86.8% |
| Hispanic | 128 | 2.3% |
| Black | 418 | 7.5% |
| Pacific Islander | 10 | 0.2% |
| Asian | 117 | 2.1% |
| American Indian/Alaska Native | 4 | 0.1% |
| Other | 33 | 0.6% |
The results of the primary endpoints of Z0010 are not yet evaluable as the clinical follow-up data continues to mature. However, we have been able to assess some of the secondary endpoints and have contributed to the published literature on the skill requirements and the surgical outcomes related to sentinel node surgery in breast cancer patients.
Participating surgeons were required to document 20 to 30 sentinel lymph node dissections performed with immediate completion axillary lymph node dissection, with a failure rate of less than 15%. Surgeons who completed a fellowship program or residency training program with training in SLND were exempt from the skill requirements as long as they had documentation of the surgical skill from their program director. At completion of patient accrual, 64.6% of participating surgeons qualified with 30 cases of SLND with ALND; 22.2% qualified with 20 cases of SLND with ALND; and 13.1% were exempted from the skills verification based on documentation from their training program. Surgeons used a combination of the blue dye and radiocolloid in 79.4% of cases, blue dye alone in 14.8% of cases, and radiocolloid alone in 5.7% of cases. Overall, surgeons achieved an SLN identification rate of 98.6% in the trial. The majority of patients had 1 (30%), 2 (34%), or 3 (19%) SLNs identified, with the average number of SLNs removed being 2.3. Patient factors that were associated with an increased likelihood of failure to identify an SLN included increasing body mass index (BMI) and increasing age (p ≤ .0001). The presence of nodal metastases, tumor stage, the number of positive lymph nodes, and the tumor histology were also assessed and were not found to be associated with a failure to identify an SLN. Surgeons with the highest patient accrual to Z0010 had the most success at identifying an SLN, and individual surgeon accrual of fewer than 50 patients was found to be associated with an increased failure rate (p ≤ .0001). The SLND technique, the specific surgeon skill qualification, and the type of institution where the procedure was performed were not associated with failure to identify an SLN.2 These patient and surgeon related factors identified in the Z0010 trial that impacted the failure rate of identifying a SLN can be used in the preoperative counseling of patients with early-stage breast cancer.
The complication rate associated with SLND was very low and less than 1% of patients required hospitalization for management of postoperative complications. There were 1791 (32%) patients reported to have at least 1 surgical side effect following breast-conserving surgery and SLND. The surgical effects reported are shown in Table 44-2. There were 2 grade 4 adverse events reported during the conduct of the trial: 1 anaphylactic reaction to the blue dye and 1 hypoxic event which was thought to be possibly attributable to study intervention. The most common effect noted at 6 months of follow-up was axillary paresthesias; 8.6% (307 of 3573) of patients were noted to have axillary paresthesias with 92% reported as mild. Decreasing age and the use of radiocolloid alone were significantly associated with the incidence of paresthesias.
| Mild | Moderate | Severe | |
|---|---|---|---|
| Allergic reaction | 20 (<1%) | 20 (<1%) | 10 (<1%) |
| Axillary paresthesia | 1249 (23%) | 338 (6%) | 50 (<1%) |
| Brachial plexus injury | 76 (1%) | 17 (<1%) | 4 (0%) |
| Lymphedema | 508 (9%) | 102 (2%) | 15 (<1%) |
| Pain/bruising from BM | 253 (5%) | 36 (<1%) | 7 (<1%) |
| Maximum grade (per patient) | 1594 (29%) | 455 (8%) | 70 (<1%) |
In comparison to the presurgical assessment, only 3.8% of patients (of 3071 assessed) had decreased range of motion of the ipsilateral upper extremity and 67% of these patients had a deficit that was <20 . In multivariate analysis, use of radiocolloid alone for the mapping procedure was the only factor that was significantly associated with decreased range of motion. Arm measurements were taken at 10 cm proximal and distal to the medial epicondyle and were compared with the presurgical measurements and the contralateral upper extremity in order to assess lymphedema. A change in arm circumference of greater than 2 cm from the presurgical measurement and compared to the contralateral arm was defined as evidence of lymphedema. At 6 months following SLND, arm measurement data were available for 2904 patients and of these, 7% had lymphedema.3 Factors identified on multivariate analysis that were significantly associated with the incidence of lymphedema included increasing age and increasing BMI.
The primary objective of Z0010 was to estimate the prevalence and to evaluate the prognostic significance of sentinel node and bone marrow micrometastases detected by immunohistochemistry and immunocytochemistry. Twenty-four percent of patients had at least one positive SLN by H&E staining. The incidence of occult metastases within the bone marrow and sentinel nodes will be reported when the clinical data are mature enough to assess the primary endpoint. In the interim, laboratory studies using Z0010 specimens have been performed in order to examine occult metastases within these compartments. Evaluation of occult metastases in the bone marrow compartment has provided the first ever evidence of the existence of a putative stem cell–like phenotype within disseminated tumor cells in the bone marrow of patients with early-stage breast cancer (CD44+/CD24-low). The Cote lab used spectral imaging on bone marrow aspirates from Z0010 participants and evaluated the cells for hematoxylin, CD44, and cytokeratin. A total of 100 slides from clinical samples on 50 patients previously categorized as cytokeratin positive were analyzed. Of these samples, 100% of repeat cytokeratin-positive cases (47 patients) had cells with the CD44+/CD24-phenotype, suggesting putative breast cancer stem cells in the bone marrow. The mean prevalence of these cells in patients with a positive bone marrow was 72%, a substantially higher percentage than has been reported in primary tumors (<10%).4 This is an important observation since stem cells are believed to be responsible for treatment failures in breast cancer patients. The identification of breast cancer stem cells in the bone marrow provides an opportunity for further characterization of these cells and ultimately an understanding of their resistance to standard therapies.
The prospective study of SLN surgery in early-stage breast cancer patients in Z0010 provides excellent short- and long-term data specific to the morbidities and disabilities associated with SLN surgery. Current results suggest low rates of local recurrence and modest rates of complications and 6-month disabilities. The data from clinical follow-up of the patients enrolled in Z0010 are under analysis for local, regional, and distant recurrence rates. It is anticipated that the presence of occult metastases in the sentinel nodes and the bone marrow will be highly prognostic in this early-stage breast cancer population. If this hypothesis is proved true when the clinical data are matched with the bone marrow and sentinel lymph node data, this would support the development of future trials using the results of bone marrow ICC and sentinel node IHC to stratify patients into high-, intermediate-, and low-risk groups.
ACOSOG Z0011—a Randomized Trial of Axillary Node Dissection in Women with Clinical T1 or T2 N0 M0 Breast Cancer Who Have a Positive Sentinel Node
The Z0011 trial was designed to evaluate the impact of axillary dissection on the outcomes of patients with carcinoma of the breast and 1 or 2 positive sentinel lymph nodes. ACOSOG Z0011 was opened in May 1999, as a companion trial to ACOSOG Z0010 with Armando Giuliano, MD, serving as the study chair for both trials. This ACOSOG-led cooperative group trial was endorsed by the North Central Cancer Treatment Group (NCCTG) and the National Surgical Adjuvant Breast and Bowel Project (NSABP), and had participation from investigators enrolling through the Clinical Trials Support Unit (CTSU).
The objectives of ACOSOG Z0011 include
To assess whether overall survival for patients with a positive SLN without completion ALND is equivalent to (or better) than that for patients with a positive SLN undergoing completion ALND
To quantify and compare the surgical morbidities associated with sentinel lymph node dissection (SLND) plus ALND versus SLND alone
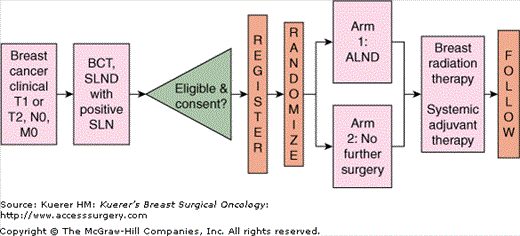
Women with clinical stage T1 or T2 N0 M0 breast cancer who underwent sentinel lymph node dissection (SLND) with breast-conserving therapy (BCT) and were found to have a sentinel node containing metastatic breast cancer, as documented on frozen section, touch prep, or permanent section evaluation by hematoxylin and eosin (H&E) staining, were eligible for this trial. The patients were randomized to 1 of 2 arms (see study schema in Fig. 44-2), where the interventions associated with these arms were
- Arm 1: Completion axillary lymph node dissection (ALND)
- Arm 2: No immediate additional axillary surgery or axillary-specific radiation
Women in both arms were to receive whole-breast radiation therapy, and systemic adjuvant therapy decisions were left to the discretion of the treating clinician. The Z0010 trial was intended to provide study participants for Z0011 by identifying women with positive SLNs; however, patients could participate in Z0011 without being registered to Z0010.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree