Testicular Cancer
EPIDEMIOLOGY
Although testis cancer is rare when viewed across the lifespan, it is the most common malignancy in men aged 20-35 years. Thanks to the high cure rate of the disease, however, it represents fewer than 5% of cancer deaths during those ages. In 2013, there were about 7,920 new cases and 370 deaths from testicular cancer. Incidence is relatively stable while mortality has been in decline. The declining mortality rate is attributed to the development of curative chemotherapy for advanced disease, improved treatment algorithms, earlier stage at presentation, and a growing proportion of seminomas relative to nonseminomas. The U.S. male lifetime risk of being diagnosed with testis cancer is between 3 and 4 in 1000. Testis cancer is exceedingly rare in African American men, whose incidence of the disease is one-fifth that of white Americans.
Major risk factors for testis cancer include cryptorchidism, and a family history or personal history of testicular cancer. Having a brother with testis cancer raises a man’s risk about 8- to 10-fold, whereas testis cancer in the father raises the son’s risk fourfold. The risk for testis cancer in men with cryptorchidism is estimated to be 10-15 times higher than in the general population, resulting in a roughly 2%-3% lifetime risk of testis cancer. Prepubertal orchiopexy is strongly recommended in men with cryptorchidism to reduce the risk of testis cancer. Men who have had testis cancer have about a 2%-3% risk of developing a second cancer in the contralateral testis.
PATHOLOGY
The vast majority of testicular neoplasms are germ cell tumors (GCTs), which include the following subcategories:
• Seminoma
• Embryonal carcinoma
• Teratoma
• Yolk sac tumor (also known as endodermal sinus tumor)
• Choriocarcinoma
Choriocarcinomas and teratomas in men are different diseases from gestational choriocarcinomas and gestational teratomas in women. In contrast, ovarian GCTs in women and pediatric GCTs are similar but not identical to testicular GCTs in post-pubescent males. For management purposes, GCTs in men are divided into pure seminomas and nonseminomas. Pure seminomas may contain no other GCT elements. Nonseminomas, in contrast, usually consist of a mixture of two or more GCT subtypes and one of these subtypes may be seminoma.
Serum tumor markers (STMs) are elevated in over half of all testis cancer patients. STMs are more likely to be elevated in advanced stage than localized disease. The following STMs all play a critical role in the diagnosis, staging, and management of testicular cancer:
• Alpha-fetoprotein (AFP) (half-life = 5-7 days)
• Human chorionic gonadotropin (HCG) (half-life = 24-36 h)
• Lactate dehydrogenase (LDH)
One critical fact is that seminomas do not produce AFP. Elevated serum AFP therefore precludes a diagnosis of seminoma regardless of the histopathology unless an alternative source for the AFP is clearly identified. Likewise, a post-orchiectomy HCG greater than 1000 IU/L would be considered inconsistent with pure seminoma by many GCT experts.
The degree of elevation of STMs following orchiectomy but prior to other treatment affects stage and prognosis. Similarly, a slower than expected rate of decline of AFP and HCG during chemotherapy is associated with a worse prognosis. Persistently elevated STMs following orchiectomy indicate the presence of metastatic disease (unless a compelling alternative explanation is identified), while rising STMs are often the earliest indication of relapse.
GCTs are not the only cause of elevated AFP, HCG, and LDH. Elevations of AFP are seen in hepatocellular carcinoma, hepatitis, and cancers of the stomach, colon, rectum, and other gastrointestinal sites. AFP can be elevated due to hepatotoxicity from chemotherapy, so elevations of AFP at the conclusion of treatment cannot always be interpreted as indicative of residual disease or relapse. Elevations of HCG are seen in biliary, pancreatic, and many other cancers, but in non-GCT neoplasms, the elevation is typically mild. Hypogonadism resulting in elevated serum gonadotropins can result in elevations of serum HCG assays due to assay cross reactivity with luteinizing hormone and due to pituitary production of HCG. In such a scenario, the elevation of HCG should resolve with supplemental testosterone. Elevations of LDH are seen in a host of cancers and other diseases, including lymphoma, liver disease, myocardial infarction, and other conditions associated with cell death.
DIAGNOSIS AND STAGING
Testis cancer typically presents with a painless or painful testicular mass. Less common presenting symptoms include gynecomastia, gynecodynia, testicular atrophy, infertility, and, in advanced stage disease, back pain, supraclavicular adenopathy, thromboembolic events, or respiratory symptoms. If a testicular tumor is suspected from the history and/or physical examination, a scrotal ultrasound should be ordered to evaluate both testicles. If the ultrasound indicates that a tumor is present, the following steps should be performed:
• Measure serum AFP, HCG, LDH
• Radical/inguinal orchiectomy
• CT scan of the abdomen and pelvis
• Chest x-ray if the CT of the abdomen/pelvis and serum tumor markers are normal
• Chest CT if the CT of the abdomen/pelvis shows nodal or visceral metastases or if post-orchiectomy STMs are elevated
• If any STM was elevated before orchiectomy, recheck STMs after orchiectomy
• Brain MRI or CT in patients with post-orchiectomy AFP or HCG >5000 or signs or symptoms intracranial metastases
 STAGING
STAGING
Testicular cancer divides into three stages:
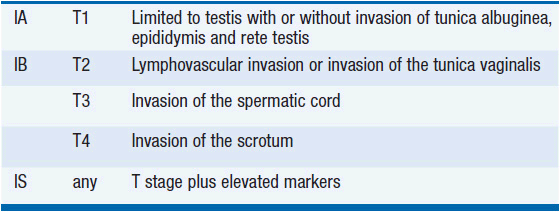
I. Limited to the testis, spermatic cord, and scrotum (Table 43-1)
II. Metastatic disease to retroperitoneal and/or pelvic lymph nodes and STMs normal or mildly elevated (Table 43-2)
III. Regional nodal metastases plus moderately to highly elevated STMs and/or visceral or distant nodal metastases
Although patients with persistently elevated STMs following orchiectomy are technically labeled stage I if there is no radiographic evidence of metastatic disease (stage IS), they are treated as stage III patients for presumed micrometastatic disease.
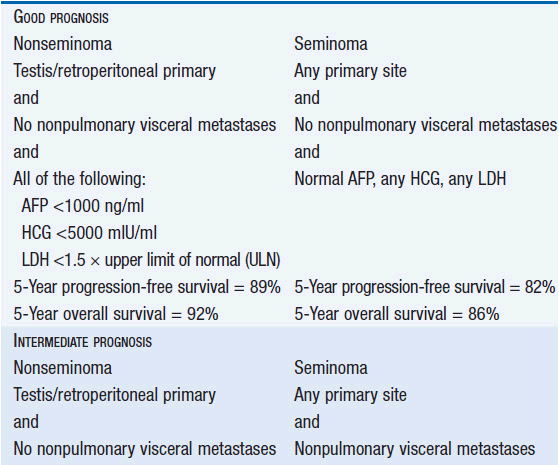
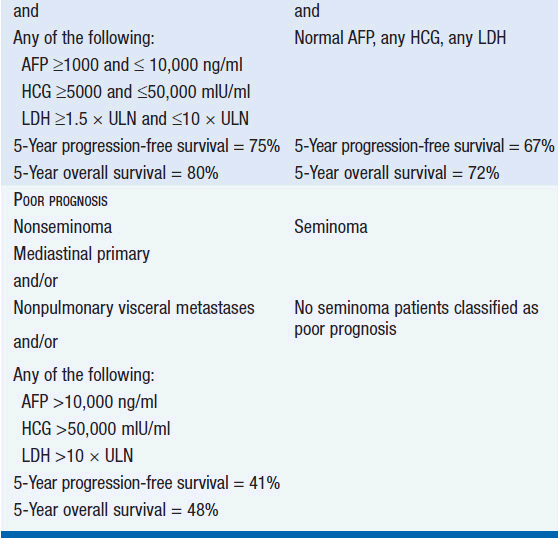
Stage III is subdivided based on the level of STMs and the location of metastases. Treatment decisions about advanced stage disease are based on the International Germ Cell Consensus Classification system that divides patients into risk categories (Table 43-3) (1).
TREATMENT
Treatment for testis cancer that includes chemotherapy, para-aortic or pelvic radiation therapy, or retroperitoneal lymph node dissection can render men infertile. Men who wish to preserve their fertility should be advised to bank semen prior to treatment. Men receiving bleomycin chemotherapy as part of their treatment must be advised to alert health-care providers to this fact in the future if they undergo surgery so that perioperative precautions can be taken. These include minimizing exposure to supplemental oxygen and IV fluids during surgery and the perioperative period.
 STAGE I AND II SEMINOMAS
STAGE I AND II SEMINOMAS
Stage I Seminoma
Stage I seminomas carry an outstanding prognosis with fewer than 1% of patients expected to die as a result of the disease. There are three management options for these patients and they result in indistinguishable long-term survival rates (2):
• Surveillance
• Single agent carboplatin chemotherapy
• External beam radiation therapy
Each option has distinct risks and benefits.
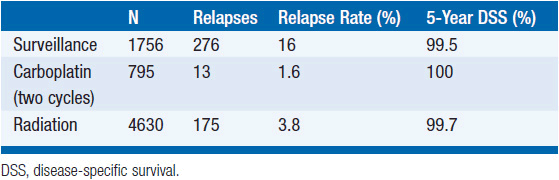
Numerous studies have reported that surveillance produces survival rates indistinguishable from those associated with radiation therapy and carboplatin chemotherapy, although no randomized controlled trials of surveillance have been published. The relapse rate for stage I patients managed with surveillance following orchiectomy is about 18% compared to 4% after radiation therapy. The vast majority of relapsing surveillance patients can be cured with radiation therapy at the time of relapse while almost all of the others can be cured with chemotherapy. Risk factors for relapse during surveillance include a large tumor (e.g., >4 cm) and invasion of the rete testis (3). Risk of relapse is about 10%, 16%, and 32% for men with zero, one, or both risk factors, respectively. A common surveillance schedule is to perform a physical examination, chest x-ray, serum tumor marker measurement, and an abdominopelvic CT scan at the following intervals:
• Years 1-3: every 4 months
• Years 4-5: every 6 months
• Years 6-10: annually
An alternative to surveillance is carboplatin chemotherapy given as either one or two cycles at a dose of an AUC of 7. A randomized trial comparing a single dose of carboplatin to external beam radiation reported no difference in relapse rate (4). Studies of two cycles of carboplatin have reported lower relapse rates of about 2% (5, 6). Very limited long-term follow-up for patients treated with carboplatin is available, but disease-specific survival in reported series is 100%. Little is known about the hypothetical risk of late relapse and late toxicity (Table 43-4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree