Testicular Cancer
 EPIDEMIOLOGY
EPIDEMIOLOGY
Testicular cancer is the most common malignancy among young men in North America and most Western European Countries. More than 95% of testicular cancers are germ cell tumors, either seminomas or nonseminomas. Seminomas are most commonly diagnosed between the ages of 30 and 34 years, whereas nonseminomas are usually diagnosed 5 to 10 years earlier. It is estimated that approximately 8,300 new cases of testicular cancer are diagnosed annually in the United States, with 350 deaths.1
There is marked variation in incidence worldwide. The highest incidence occurs in Northern and Western European countries (up to 9 per 100,000) and the lowest in Asian and African populations (<1 per 100,000).2,3 In North America, incidence rates are highest among Whites and lowest among Blacks, with an overall increase in age-adjusted incidence rate in the United States of 72% between 1975 and 2004.4,5 A similar increased incidence has been reported in Europe and Australasia.6–8 Despite the increased incidence of both seminoma and nonseminoma, there has been a significant reduction of mortality and improvement in survival. The most dramatic reduction in mortality occurred in the 1970s with the introduction of cisplatin-based chemotherapy.9,10 The 10-year survival for seminoma increased from 81% in 1970–1979 to 95% in 2000–2002; that for nonseminoma increased from 54% to 92%.11
A history of undescended testicle has long been recognized as a risk factor for the development of testicular cancer. The testes develop in the abdomen before birth and normally descend into the scrotum. In cases of testicular maldescent, normal germ cell development is impaired and the testicle is prone to malignancy. There is also an increased risk of malignancy in the normally descended contralateral testicle. The relative risk is estimated to be 6 in the undescended testicle and 2 in the contralateral testicle.12 The mechanism of the increased risk is unclear. It is hypothesized that a common etiologic agent predisposes both to testicular maldescent and subsequent malignancy. It is also possible that the maldescended testicle is subject to a hostile and malignancy-inducing environment.
The temporal and geographic variation in incidence rates strongly suggests environmental etiologic factors. Male infertility is associated with an increased risk of testicular cancer, and infertile men with abnormal semen analyses have a 20-fold greater risk of testicular cancer than the general population.13 The increasing incidence has paralleled decline in semen quality over the past several decades.14,15 This has led to the hypothesis that poor semen quality, testicular cancer, cryptorchidism, and hypospadias represent a testicular dysgenesis syndrome as a result of disruption of gonadal development during embryonal development.16 Various exogenous hormonal disruptors (e.g., exogenous estrogens) have been proposed, although with no conclusive evidence implicating one particular agent.
Approximately 2% of patients report a positive family history. Sons of cases have a 4- to 6-fold increase in risk and brothers an 8- to 10-fold increase.17 Several candidate genes have been proposed to explain familial testicular cancer; however, it is likely that no single genetic change explains most familial cases.
In summary, germ cell tumors are thought to arise in testes predisposed to the development of malignancy owing to a combination of familial predisposition and intrauterine hormonal imbalance, later compounded by environmental factors and manifested by impaired spermatogenesis.
 PATHOLOGY
PATHOLOGY
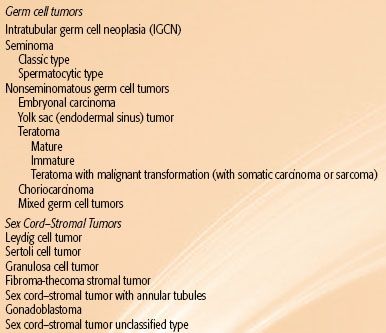
More than 95% of testicular neoplasms are germ cell tumors and are divided into seminomas and nonseminomas. Nonseminomas include embryonal carcinoma, yolk sac (endodermal sinus tumour), teratoma, choriocarcinoma, and mixed germ cell tumors. Intratubular germ cell neoplasia (IGCN) is the putative precursor of most germ cell neoplasms. Sex cord–stromal tumors make up the remaining 3% to 4% of testicular neoplasms, and most are benign (Table 67.1).
TABLE 67.1 CLASSIFICATION OF TUMORS OF THE TESTIS
 CLASSIFICATION OF TESTICULAR TUMORS
CLASSIFICATION OF TESTICULAR TUMORS
Germ Cell Tumors
Intratubular Germ Cell Neoplasia
IGCN is found adjacent to invasive germ cell tumors in >95% of cases. It is also found in all clinical groups known to be at high risk for testicular cancer development: cryptorchidism (2% to 4%), infertility (1%), ambiguous genitalia (25%), and contralateral testes of patients with testicular cancer (5%).18 IGCN is characterized by seminiferous tubules showing decreased spermatogenesis in which the normal constituents of the tubules are replaced by abnormal germ cells with the appearance of seminoma cells. These cells stain strongly for placental alkaline phosphatase (PLAP), whereas normal germ cells are negative. IGCN has a 50% risk of developing into an invasive germ cell tumor within 5 years. It is hypothesized that the cells originate from primordial germ cells early during embryogenesis, possibly owing to an excess of estrogens. They likely remain within the seminiferous tubules in a dormant stage until puberty when replication begins, possibly as a consequence of raised sex hormone levels. Transition to an invasive germ cell tumor then occurs.
 SEMINOMA
SEMINOMA
Classic Type
Seminoma accounts for >50% of all germ cell neoplasms. Serum level of human chorionic gonadotropin (HCG) is elevated in 15% to 30% of men at presentation, related to the presence of syncytiotrophoblastic cells. These may be identified in 7% of tumors on routine hematoxylin and eosin sections or by immunoperoxidase stains in 24%. Serum alpha-fetoprotein (AFP) is not elevated in pure seminoma. Grossly, seminoma is a soft tan-colored diffused multinodular mass. Focal necrosis is sometimes present. A prominent lymphocytic infiltrate is commonly seen within the fibrous stroma. More than 90% of seminomas will stain positive for PLAP.
Spermatocytic Seminoma
Spermatocytic seminoma accounts for 2% of testicular tumors. It tends to occur in an older age group at a mean age of 54 years. It is important to differentiate spermatocytic seminoma from seminoma, as the natural history and treatment is quite different. Spermatocytic seminoma is confined to the testes and is cured by orchidectomy. Metastasis is rare. The cell of origin of spermatocytic seminoma is unknown. Unlike seminoma, it does not contain glycogen and stains negative for PLAP. In fact, many authorities believe that there is little to indicate that spermatocytic seminoma is of germ cell origin.19,20
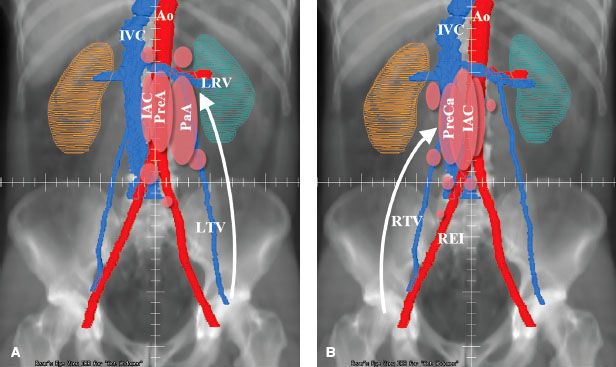
FIGURE 67.1. A: The left testis drains primarily by lymphatics along the left testicular vein (LTV) to lymph nodes inferior to the left renal vessels (LRV). Left para-aortic (PaA), preaortic (PreA), and interaortocaval (IAC) nodes are most commonly involved. Less commonly, nodal involvement may be found inferior to bifurcation of the aorta (Ao) or inferior vena cava (IVC), or superior to the renal vessels. B: Primary lymphatic drainage from the right testis (arrow) is along the right testicular vein (RTV) to precaval (PreCa) and then to interaortocaval and preaortic nodes. Less commonly, preaortic, right common iliac, or right external iliac (REI) nodes are involved.
 NONSEMINOMATOUS GERM CELL TUMORS
NONSEMINOMATOUS GERM CELL TUMORS
Nonseminomatous germ cell tumors (NSGCTs) usually contain a mixture of germ cell types (embryonal, yolk sac, teratoma, and choriocarcinoma), although tumors comprised of just one component may be found. Embryonal carcinoma is the most common component in mixed tumors and AFP- and HCG-positive cells are present in 33% and 20%, respectively. Yolk sac tumors are associated with high levels of AFP and are the most common germ cell tumors of childhood. Teratoma is not associated with elevated AFP or HCG, and both mature and immature teratomas are considered malignant with ability to metastasize. Choriocarcinoma is the least common type of pure NSGCT and is present in about 4% of mixed tumors. It is particularly aggressive, almost always metastatic at diagnosis, and associated with high levels of HCG. Seminomatous components are also common in mixed tumors. Serum markers (AFP and HCG) are elevated depending on the relative germ cell elements present.
 ANATOMY AND NATURAL HISTORY
ANATOMY AND NATURAL HISTORY
In the developing embryo, the testes originate from the genital ridge located near the second lumbar vertebra. Accompanied by their blood supply and lymphatics, they descend into the scrotum via the inguinal canal. As a result, the primary lymphatic drainage from the testis is to the retroperitoneal lymph nodes. The lymphatic vessels first drain into the collecting trunks at the hilum of the testicle. These lymphatic trunks accompany the testicular artery, vein, and spermatic cord through the internal inguinal ring and then continue proximally to the retroperitoneal lymph nodes. The retroperitoneal lymph nodes are situated anterior to the T11 to L4 vertebral bodies, concentrated at the L1-3 level. On the left, the lymphatics drain primarily into the preaortic and para-aortic lymph nodes around the left renal hilum and from there to the interaortocaval nodes (Fig. 67.1A). On the right, the first nodes involved are usually in the precaval or interaortocaval region, followed by the preaortic lymph nodes (Fig. 67.1B). Contralateral spread is mainly seen with right-sided tumors and rarely with left-sided tumors.
From the retroperitoneal nodes, the lymph drains into the cisterna chyli, thoracic duct, posterior mediastinum, and left supraclavicular fossa. The thoracic duct drains into the left subclavian vein in the left supraclavicular region. In 5% to 10% of patients, drainage into the right supraclavicular area can occur.
Aberrant lymphatic drainage may occur in the event of previous scrotal or inguinal surgery. Hernia repair alters the drainage of the testicle. The testicular lymphatic vessels anastomose with the regional lymph vessels, resulting in drainage into the ipsilateral inguinal and iliac lymph nodes. In addition, the testicular trunks may abandon the spermatic vessels at the internal inguinal ring and pass posteriorly and superiorly into the external iliac lymph nodes. The scrotum drains directly into the inguinal and external iliac lymph nodes.
Seminoma has an orderly and predictable pattern of spread. Locoregional lymphatics are the first site of metastatic disease. From the retroperitoneal lymph nodes, seminoma spreads proximally to involve the next echelon—the mediastinal lymph nodes—and then the supraclavicular lymph nodes. Very occasionally, metastases from retroperitoneal lymph nodes can drain directly via the thoracic duct to the supraclavicular fossa, resulting in supraclavicular metastases in the absence of mediastinal disease. Hematogenous metastases are rare in pure seminoma, being much more common with NSGCT. Lung is the most common site of distant disease, although bone, liver, and brain may also be involved.
 CLINICAL PRESENTATION AND DIAGNOSTIC WORKUP
CLINICAL PRESENTATION AND DIAGNOSTIC WORKUP
A testicular tumor usually presents as a painless swelling in the scrotum, although pain, heaviness, and tenderness at presentation are not uncommon. Disease in the lymph nodes of the retroperitoneum may produce back pain or abdominal swelling. Widely disseminated parenchymal disease in lungs, liver, bone, or brain is rare but, if present, may produce systemic symptoms. Gynecomastia is a rare presentation of embryonal carcinoma that may be seen in association with the very uncommon sex cord–stromal tumors. Occasionally, patients present with metastatic germ cell malignancies diagnosed by biopsy or elevated levels of serum tumor markers without evidence of a palpable mass in the testis. Occult primary disease in the testis is often detected by testicular ultrasound. If there is no evidence of a primary tumor in the testis, a diagnosis of an extratesticular germ cell tumor—usually mediastinal, retroperitoneal, or pineal—may be made.
TABLE 67.2 DIAGNOSTIC WORKUP FOR TUMORS OF THE TESTIS

 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
The tests necessary to evaluate patients with testicular cancer are listed in Table 67.2. A complete history should be taken, including information about previous inguinal or scrotal surgery, cryptorchidism, retractile testes, and orchidopexy. The physical examination should pay special attention to possible sites of lymph node metastases. The contralateral testis should be examined clinically. The presence or absence of gynecomastia is an important observation. If testicular tumor is suspected, testicular ultrasound should be performed. This usually demonstrates a solid mass within the testis, often with associated testicular microlithiasis. Radical orchiectomy through an inguinal incision is diagnostic and removes the primary tumor.
Laboratory Studies
A routine complete blood count and chemistry screen, including renal function tests, should be done. Pulmonary or renal function tests should be performed for patients who may receive bleomycin or combination chemotherapy. NSGCTs of the testes are uniquely associated with reliable serum tumor markers: the β-subunit of human chorionic gonadotropin (β-HCG) and AFP. One or both of these serum markers are elevated in 80% to 85% of patients with disseminated nonseminomatous disease. The metabolic half-life of AFP is approximately 5 days and for β-HCG is approximately 18 to 24 hours. Although β-HCG may be modestly elevated in 15% to 30% of patients with pure seminomas, usually any elevation of AFP connotes nonseminomatous disease. Serum tumor markers may be elevated in other circumstances or conditions, such as laboratory error, cross-reactivity with luteinizing hormone, marijuana use, hepatitis, or development of antibodies to the glycoproteins. Serum lactate dehydrogenase (LDH), although nonspecific, is elevated in 80% of patients with advanced testicular cancer.
If a testicular cancer is suspected, serum tumor markers should be assayed before and after orchiectomy, and interpretation of the levels of markers should take into account their metabolic half-lives. Serum tumor markers can document persistent or recurrent cancer after surgery or chemotherapy and may predict the responsiveness of nonseminomas to treatment. The level of β-HCG should decrease by ≥90% every 21 days with each successful treatment cycle of chemotherapy. A slow decline in β-HCG after treatment may imply suboptimal response to chemotherapy, permitting early implementation of salvage therapy before the development of overt resistance to chemotherapy. The decline of AFP is less predictable.
Semen analysis and banking of sperm should be considered for patients in whom treatment is likely to compromise fertility. With newer technologies, it is possible to retrieve and bank sperm even with poorer-quality sperm.
Radiographic Studies
Investigations should routinely include chest x-ray films for all patients and computed tomography (CT) of the thorax for any patient with NSGCTs of the testis. CT scans of the abdomen and pelvis should be performed to evaluate the retroperitoneal nodal areas and assess the liver. CT of abdomen and pelvis relies on nodal size to assess the retroperitoneal nodes, with a sensitivity of 40% and a specificity of 95%.21 There is considerable overlap between the size of normal and abnormal lymph nodes using a size limit of 10 to 20 mm. It therefore has limited ability to exclude the presence of disease, although enlarged lymph nodes in the appropriate clinical context (location, laterality, disease parameters) are very likely to be truly positive. Magnetic resonance imaging (MRI) appears equivalent to CT in determining the size and location of retroperitoneal adenopathy. Fluorodeoxyglucose positron emission tomography (FDG-PET) scan has a slightly higher sensitivity (66%) than CT with a comparable specificity (98%). It has little role in initial disease staging but may have a role where CT is questionable.21 It is unable to detect lesions <5 mm in size or teratomas of any size owing to their very low metabolic activity. It also has an important role in evaluating residual retroperitoneal disease following chemotherapy.
Baseline ultrasonography of the remaining testis should be performed. If the contralateral testis is atrophic and the patient is <30 years of age, then there is a 30% risk of IGCN. Biopsy of the contralateral testis may be considered in this setting.
TABLE 67.3 AMERICAN JOINT COMMITTEE ON CANCER 2010 STAGING FOR TESTICULAR CANCER

TABLE 67.4 INTERNATIONAL GERM CELL CANCER COLLABORATIVE GROUP CONSENSUS CLASSIFICATION OF METASTATIC GERM CELL CANCERA
 STAGING AND PROGNOSTIC FACTORS
STAGING AND PROGNOSTIC FACTORS
Patients are staged according to the American Joint Committee on Cancer (AJCC) criteria as indicated in Table 67.3, which incorporates features of the primary tumor (T), node (N), metastasis (M), and level of serum tumor markers (S).22 Approximately 80% of seminoma patients and 50% to 60% of nonseminoma patients have stage I disease at presentation. For stage I seminoma, tumor size and rete testis invasion are the most commonly reported predictors of recurrence. For nonseminomas, T-stage, extensive embryonal component, and lymphovascular invasion are prognostic. Age is an adverse prognostic factor for patients diagnosed with all stages of testicular cancer.23
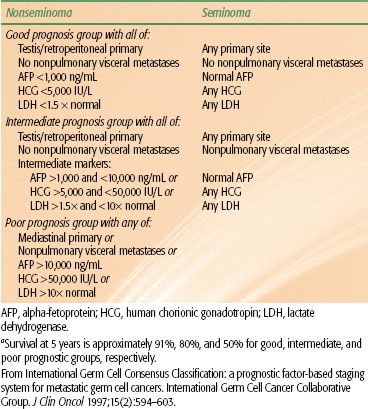
The International Germ Cell Cancer Collaborative Group (IGCCCG) developed a widely accepted classification system for patients with metastatic germ cell malignancies, which has been incorporated into the TNM system24 (Table 67.4). The prognostic classification is based on data collected on approximately 6,000 patients with metastatic germ cell tumor from 10 countries during the platinum era. The classification was internally validated as well as prospectively validated on a subsequent cohort of patients. The factors most strongly associated with a poor prognosis were mediastinal primary, nonpulmonary visceral metastases, or grossly elevated tumor markers (AFP >10,000 ng/mL, HCG >50,000 IU/L, or LDH >10 times normal). Patients with NSGCT were divided into three prognostic groups (good, intermediate, and poor prognosis) and seminomas into either good or intermediate prognostic groups (Table 67.3). The good prognosis group comprised >50% of all patients with metastatic NSGCTs and 90% of seminomas and was associated with a 5-year survival >90%. The intermediate prognosis group comprised 25% to 30% of patients and had a 5-year survival of 80%. The poor prognosis group comprised 15% to 20% of patients with NSGCT and had a 5-year survival of approximately 50%.
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
The initial management of a suspected malignant germ cell tumor of the testis consists of obtaining serum AFP and β-HCG measurements and then performing a radical (inguinal) orchiectomy with division of the spermatic cord at the internal inguinal ring. Historically, it had been thought that scrotal violation (transscrotal orchiectomy, open testicular biopsy, or fine-needle aspiration) compromised prognosis. Scrotal violation is associated with a slight increase in local recurrence rate compared with inguinal orchiectomy (2.9% vs. 0.4%, respectively) but is not associated with any difference in distant recurrence rates or overall survival.25 Orchiectomy is both diagnostic and therapeutic. Further management depends on pathologic diagnosis and the stage and extent of disease. Radiation treatment plays a significant role in the management of seminomas. Nonseminomatous tumors are generally managed by cisplatin-based combination chemotherapy and/or surgical resection.26 A detailed discussion of the management of nonseminomatous tumors is beyond the scope of this chapter.
 SEMINOMA
SEMINOMA
Stage I
Patients with stage I seminoma have a risk of relapse of approximately 20%. Either adjuvant radiotherapy or adjuvant chemotherapy with single-agent carboplatin is associated with a disease-free survival >95% and disease-specific survival approaching 100%.27 Surveillance, with treatment at time of relapse, is associated with a similar survival outcome and allows 80% of patients to avoid morbidity of treatment.28
TABLE 67.5 PATTERNS OF RECURRENCE FOR STAGE I SEMINOMA MANAGED BY SURVEILLANCE
Surveillance
Surveillance has become the management strategy of choice for most men with stage I seminoma. A survey of Canadian radiation oncologists in 2006 revealed that 56% felt surveillance was the preferred management.29 Kollmansberger et al.30 document increasing use of surveillance for managing patients with stage I seminoma in a population-based cohort from British Columbia, Canada, and Oregon. Between 1999 and 2008, the proportion of patients receiving adjuvant radiotherapy decreased from 50% to 9%, and the proportion being managed by surveillance increased from 47% to 87%.
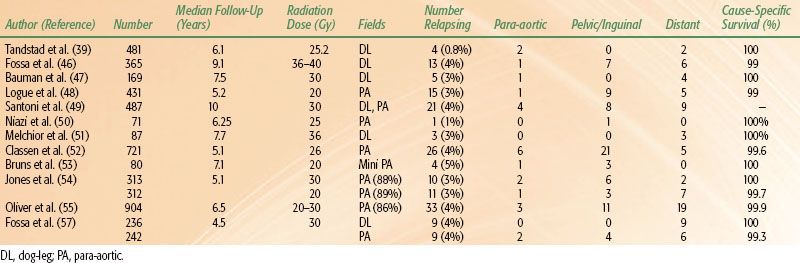
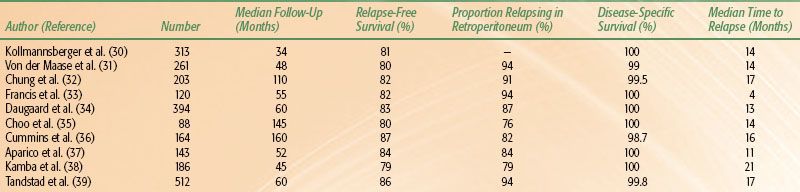
The reported series (Table 67.5) include >2,400 patients, and all report similar rates, timing, and patterns of recurrence.30–39 When managed by surveillance, 20% of patients will relapse at a median time of around 14 months. In the series of 394 patients from Copenhagen, Denmark, median time to relapse was 13 months, with 87% occurring within the first 2 years and only 2% beyond 5 years. Choo et al.35 reported similar results from Toronto, with a median time to relapse of 13.6 months and only 2 of 88 patients relapsing after 5 years. In the Princess Margaret Hospital (PMH) series, the risk of relapse between 5 and 10 years was 4%. Given the small event rate in the individual reports, Warde et al.40 performed a pooled analysis from four series (PMH, Royal Marsden Hospital [RMH], Royal London Hospital, and the Danish Testicular Cancer Study Group). Individual patient data on 638 stage I seminoma patients managed by surveillance was obtained. With a median follow-up time of 7 years, the 5- and 10-year relapse-free rates were 82.3% and 78.7%, respectively. Most relapses (69%) occurred within the first 2 years of surgery, whereas 7% relapsed beyond 6 years. The 5-year cause-specific survival was 99.3%. This data is consistent with large population-based reports from Kollmansberger et al.30 and Tandstad et al.39
The predominant site of relapse is in the retroperitoneum (76% to 94%). Approximately 5% to 15% of patients relapse in the mediastinum or lungs, with inguinal relapse reported in 3% to 11%, usually only after previous scrotal interference. Some variability is reported in management at time of relapse. Traditionally, most (74% to 82% of patients in the older series) were initially managed by radiation therapy, with a second relapse occurring in about 10% (6% to 16%). Second relapse almost always occurred at distant sites with a 90% to 95% rate of successfully salvage with chemotherapy. More recent series describe a greater use of chemotherapy as initial salvage treatment. In the population-based study from the Swedish Norwegian Testicular Cancer Study Group, cisplatin-based combination chemotherapy as salvage treatment was given to 89% of relapsing patients,39 with further relapse in only 1 of 58 patients. Kollmansberger et al.30 report the use of salvage chemotherapy in 68% of relapsing patients in the British Columbia and Oregon Testis Cancer Program database. No further relapse occurred in the 32 patients managed by chemotherapy, whereas relapse occurred in 3 of 15 patients who received salvage radiotherapy. Although salvage chemotherapy is effective, it is associated with greater toxicity than salvage radiotherapy.
In the pooled analysis,40 tumor size, rete testis invasion, and lymphovascular invasion were predictive of relapse on univariate analysis. On multivariate analysis, tumor size (hazard ratio [HR] 2.0) and rete testis invasion (HR 1.7) remained significant. The relapse-free rate decreased from 87.8% for tumors <4 cm without rete testis invasion to 68.5% for tumors >4 cm with rete testis invasion. These risk factors, however, have not been validated on subsequent patient cohorts. Tyldesley et al.41 also noted the importance of size >4 cm and rete testis invasion, reporting a 5-year relapse-free rate of 86%, 71% and 50% in patients with no risk factor, one risk factor, or both risk factors, respectively. Choo et al.35 reported a reduction in 10-year relapse-free rate from 86% to 52% with rete testis invasion. However, the large population-based report from Tandstad et al.39 failed to identify tumor size, vascular invasion, patient age, or elevated HCG as prognostic factors. Rete testis invasion was not routinely documented.
Given the varying relapse risk with time and patterns of recurrence, a reasonable surveillance policy involves four monthly assessments in the first 2 years, six monthly assessments in years 3 and 4, and annual assessments in years 5 to 10.42 Assessment should include physical examination and CT scan of abdomen and pelvis. Although chest x-ray is also usually included, in the large series of 527 patients from PMH, all but one of the relapses was detected on abdominopelvic CT scan.43 No relapse was detected on chest x-ray alone, and the authors suggest omitting routine chest imaging from the follow-up schedule. The use of low-dose CT44 imaging or MRI is being investigated to further reduce long-term radiation exposure.
Surveillance is a slightly more costly approach to management than adjuvant nodal irradiation owing to the increased number of radiologic investigations.33–45 Surveillance should be contemplated and conducted only with a compliant patient and with an understanding that because of the risk of late relapse, the patient should be monitored for at least 10 years. The survival rate of 99.5% from the large surveillance series indicates that this therapeutic option produces a result equivalent to that achieved with immediate adjuvant treatment and is a safe and effective alternative management, provided that guidelines are followed.
TABLE 67.6 RATE OF RELAPSE AND LOCATION FOR STAGE I SEMINOMA MANAGED BY ADJUVANT RADIOTHERAPY