Odds ratios
P value
Local relapse
0.73
0.02
Distant relapse
0.67
0.0001
Overall survival
Doxorubicin
0. 84
0.09
Doxorubicin + ifosfamide
0.56
0.01
Table 31.2
Phase III study evaluating the efficacy of adjuvant chemotherapy in the treatment of soft tissue sarcoma
Study | Regimen | Patients (n) | Results |
|---|---|---|---|
Frustaci et al. | Doxorubicin: 120 mg/sqm | 104 | >DFS |
Ifosfamide: 9 g/sqm + GCSF | >OS | ||
EORTC 62771 | Doxorubicin: 50 mg/sqm | 468 | >DFS |
DTIC: 400 mg/sqm gg 1–3 | =OS | ||
Cyclophosphamide: 500 mg/sqm | |||
Vincristine: 1.5 mg/sqm | |||
EORTC 62931 | Doxorubicin: 74 mg/sqm | 351 | =DFS |
Ifosfamide: 9 g/sqm + GCSF | =OS |
Among the different antineoplastic agents available in the treatment of soft tissue sarcoma in the metastatic setting, doxorubicin- and ifosfamide-based regimens are still the most widely used. Doxorubicin is an anthracycline which was introduced in the management of metastatic soft tissue sarcoma in 1970. The administration of doxorubicin single agent as a first-line treatment (50–75 mg/m2 every 3–4 weeks, given both as bolus and as continuous infusion) is associated to a response rate of almost 20 %, and it is usually well tolerated. Unfortunately, doxorubicin use is however limited by its well-known cardiotoxicity, which is related to cumulative dose (higher than 550 mg/mq) and probably also to the administration manner (lower with continuous infusion and higher with bolus) [14]. A possible alternative could be represented by pegylated liposomal doxorubicin, which seems to share the same efficacy of the non-pegylated formulation with a better tolerability profile: in fact, a phase II trial by EORTC pointed out how the incidence of myelosuppression, alopecia and left ventricle dysfunction is significantly lower in patients treated with pegylated liposomal form [15]. Ifosfamide is an alkylating agent which has been introduced in the treatment of metastatic soft tissue sarcoma in 1980, and its administration, at the dose of 9 g/m2 every 3 week, is associated with a 20 % response rate; better results are obtained if it is used as a first line. Comparing doxorubicin- and ifosfamide-based treatment, no differences have been identified in terms of PFS and OS, but the tolerability profile seems to be better when doxorubicin is used [10]. A phase III study in adults with advanced soft tissue sarcomas which aimed to compare the objective regression rates, toxicity and survival of patients receiving doxorubicin alone and ifosfamide plus doxorubicin proved how the combination therapy reduced a significantly higher regression rate than did doxorubicin alone (34 % vs 20 %, P = 0.03), but it was also associated to a significantly more intense myelosuppression [16].
Trabectedin (ET743 or Yondelis) is a marine-derived antineoplastic molecule isolated from the Caribbean tunicate Ecteinascidia turbinata and currently produced synthetically. In Europe, today, trabectedin given as monotherapy (1.5 mg/mq as a 24-h continuous infusion every 3 weeks) is the only agent approved as a second line in the treatment of advanced soft tissue sarcoma after the failure of anthracycline- and ifosfamide-based regimens and received orphan drug status from the European Commission for the treatment of ovarian cancer in October 2003. The US FDA granted trabectedin orphan drug status both for soft tissue sarcoma and ovarian cancer. Trabectedin mechanism of action is today still not completely understood. What we know today is that, thanks to its structure, trabectedin can bind to DNA minor groove and alkylated guanine at the N2 position, bending DNA toward the major groove. Three nonrandomised phase II studies assessed the efficacy of trabectedin in patients with heavily pretreated, advanced/metastatic soft tissue sarcoma: the results from those studies benefit associated with trabectedin therapy in terms of overall response rate (4–8 %), 6-month progression-free survival (24–29 %) and overall survival (9.2–12.8 months); this benefit was particularly evident in the treatment of liposarcomas and leiomyosarcomas compared to other soft tissue sarcoma subtypes [17–19]. Trabectedin is also associated with an acceptable tolerability profile: the most significant side effects observed were grade 3 or 4 transaminase increase (26–50 %) and neutropenia (34–61 %). As for trabectedin hepatic effects, plasma liver enzyme level (transaminases, bilirubin, alkaline phosphatases and 5′-nucleotidase) should be checked before each course, because patients with any baseline alterations have a significant higher probability of developing a severe liver toxicity. The risk of developing grade 3–4 toxicities seems to be strongly reduced through the patients’ premedication with dexamethasone (4 mg per os bid 24 h before therapy). A study by Grosso et al. showed how elevation of transaminases, neutropenia and thrombocytopenia incidence was significantly lower in patients with prior dexamethasone (2, 2 and 0 %) and then in those who have not been previously premedicated (34, 24 and 25 %, respectively) [20].
Recently, particular attention has been dedicated to the evaluation of the association between gemcitabine and docetaxel in the treatment of soft tissue sarcoma, which seems to be promising. A phase II trial conducted by Maki et al. in 2007 randomised 122 patients to receive gemcitabine alone (1,200 mg/m2 days 1 and 8) or gemcitabine (900 mg/m2 days 1 and 8) plus docetaxel (100 mg/m2 day 8) every 21 days: in those patients who received the combined regimen, the radiological response rate resulted higher than in those treated with gemcitabine alone (16 % vs 10 %), as also PFS (6.2 vs 2.6 months) and OS (18 vs 11.2 months). The toxicity, even if higher in the gemcitabine/docetaxel arm, was acceptable [21]. The association between gemcitabine and docetaxel has been found to be tolerable and highly active especially in leiomyosarcoma, for which a response rate of 57 % has been recorded [22].
In the management of MCC, surgery remains today the primary treatment modality. The role for systemic therapy today is still extremely marginal, and the data today available on the efficacy of standard chemotherapy in the treatment of this extremely aggressive disease are disappointing. The most common regimen used in the treatment of MCC is cis-platinum (or carboplatinum) and etoposide, both in the adjuvant and metastatic settings. While a role for adjuvant radiotherapy is increasingly supported by retrospective data, several recent studies have failed to demonstrate a benefit for MCC from adjuvant chemotherapy, whose use should be on the contrary strictly limited to clinical trials. A phase II study conducted by Allan et al. pointed out how the administration of adjuvant therapy in patients with stage II, nodal-positive MCC was associated with a detrimental effect on survival: in fact, the 4 years’ survival in those patients who received an adjuvant treatment was 42 % compared with the 60 % of those who underwent surveillance [9]. Moreover, different studies which assessed the safety of an adjuvant treatment for MCC have proved how chemotherapy is associated with a significant treatment-related mortality (3.4 %) [23] and morbidity (63 % of patients experienced serious skin toxicity and 40 % were admitted for neutropenia) [24]. Data about the use of chemotherapy in the metastatic setting show how, despite the chemosensitive disease, the impact of the treatment on the survival is minimal. In fact, the response rate for MCC metastatic patients treated with standard chemotherapy is 57 %, but the median overall survival was only 9 months and the 3 years overall survival 17 %. Of note, a high rate of toxic death during first-line treatment (n = 7.7 %) was reported for these patients. A further trial, which aimed to specifically evaluate the efficacy of the association between cisplatin (or carboplatin) and etoposide, confirmed the previous observations according to which MCC appears to be chemosensitive but can progress rapidly with fatal outcomes [25].
Unlike soft tissue sarcoma and MCC, the identification of a specific pathway which seems to drive the growth of DFSP has led to the development of a target therapy with a well-recognised activity. As discussed before, nearly 90 % of DFSP are characterised by the presence of a rearrangement of chromosomes 17 and 22, which can be represented both by translocation t(17;22) (q22;q13) and by a supernumerary ring chromosome containing several copies of the t(17;22) breakpoint region. This genetic aberration results in the fusion of the COL1A1 and PDGFB genes, with PDGFB passing under the control of COL1A1 promoter which is more transcriptionally active than its own promoter. Upregulated PDGFB acts as a ligand for both PDGFRα and PDGFRβ, and it causes an autocrine/paracrine stimulation which is thought to be a key point in DFSP pathogenesis. It is important to remember that 8–10 % of DFSB do not present the t(11;22) translocation: in this case, other pathways not still recognised probably drive the tumour growth (Fig. 31.1) [26]. This pathogenic mechanism represents the rational according to which imatinib mesylate was tested in the treatment of DFSP. Apart from many case reports today present in the literature, an important experience was published in 2005 by McArthur et al. who analysed the efficacy of imatinib, at the dose of 400 mg twice a day, in the treatment of six patients with locally advanced DFSP and two patients with metastatic disease. Of interest, all the patients with t(11;22) translocation reported a partial response, while one metastatic patient, in whose DFSP the translocation was not present, progressed [27]. These data have led to approval of imatinib by the US Food and Drug Administration for treating locally advanced DFSP. Although wide surgical excision remains standard care, patients with locally advanced disease not suitable for surgical excision can be treated with the PDGFR inhibitor imatinib, which sometimes allows residual DFSP to be surgically excised. Because tumours lacking the t(11;22) translocation may not respond to imatinib treatment, DFSP molecular analyses should be always performed before starting a systemic therapy.
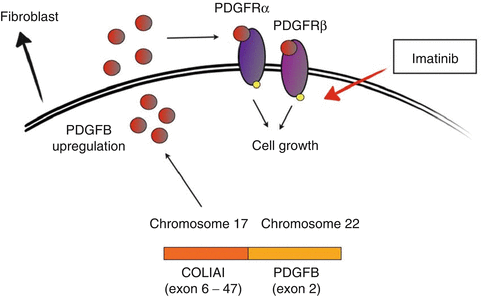

Fig. 31.1
DFSP pathogenesis and imatinib mesylate mechanism of action
Future Considerations
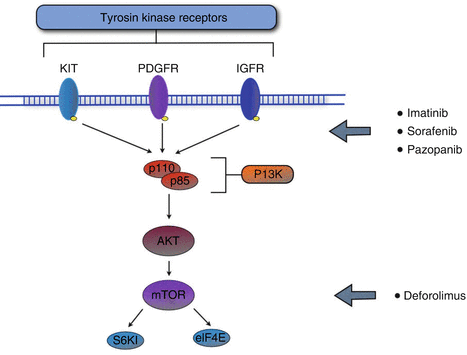
Given the poor results obtained with standard chemotherapy, currently many ongoing clinical trials are investigating the efficacy of alternative systemic therapy in the treatment of metastatic soft tissue sarcoma. Of interest, promising results have been obtained with the inhibition of the PI3K/Akt/mTOR pathway (Fig. 31.2). The PI3K/Akt/mTOR pathway has been found to be deregulated in many different human sarcomas, including leiomyosarcoma, rhabdomyosarcoma and GIST [28], underlining its importance in the promotion of cancer cell growth and survival. mTOR (mammalian target of rapamycin) appears to be regulated by the insulin-like growth factor (IGF) through the PI3K/Akt pathway, while mTOR downstream targets are mainly represented by S6K1 (ribosomal kinase) and 4E-BP1 (translational initiation factor) [29]. Rapamycin (or sirolimus), a macrolide antibiotic isolated from a strain of Streptomyces hygroscopicus, is an mTOR inhibitor routinely used in immunosuppressive regimen, which has shown efficacy in the treatment of iatrogenic [30, 31] and classic [32, 33] Kaposi’s sarcoma. Among the mTOR inhibitors which are currently in clinical development as anticancer agents, AP23573 (deforolimus), developed by ARIAD Pharmaceuticals Inc. both in intravenous and oral formulations, has recently demonstrated a single-agent activity in a broad range of soft tissue sarcoma in a phase II trial: in 212 patients affected by advanced soft tissue and bone sarcoma treated with deforolimus, the clinical benefit response rate (CBR, defined as a complete or partial response or stable disease for at least 16 weeks) was found to be 29 %, and the overall survival (OS) was 67.6 weeks. On the basis of these promising results, a multinational, phase 3 trial (SUCCEED: sarcoma multi-center clinical evaluation of the efficacy of deforolimus) has been conducted in order to investigate the efficacy and safety of oral deforolimus when administered as maintenance therapy to patients with metastatic soft tissue or bone sarcomas who have had a favourable outcome to chemotherapy. The results from the SUCCEED trial showed an improvement in PFS by 21 % and in terms of overall survival, a trend in favour of deforolimus (88 vs 77.8 weeks) [34]. Among the new therapy under development in the treatment of soft tissue sarcoma, encouraging data have been also obtained through the inhibition of tyrosine kinase receptors (VEGFR, PDGFR, c-Kit, Raf), whose activation is known to be involved in angiogenesis, cell proliferation and apoptosis (Fig. 31.2). Among small molecule tyrosine kinase inhibitors (smTKI), the most promising results in the treatment of soft tissue sarcoma have been obtained with pazopanib and sorafenib. Pazopanib, developed by GlaxoSmithKline Inc., is an oral, second-generation multi-targeted tyrosine kinase inhibitor that targets PDGFR, VEGFR and c-kit, key proteins responsible for tumourigenesis and tumour progression. A recent phase II trial [35], which enrolled 142 patients affected by adipocytic STS, leiomyosarcomas, synovial sarcomas or other soft tissue sarcoma types to be treated with pazopanib, proved an increase of PFS at 12 weeks only in the leiomyosarcoma cohort (44 %), in the synovial sarcomas (49 %) and in the other soft tissue sarcoma types (39 %). On the basis of these results, a phase III trial is ongoing to investigate whether treatment with pazopanib improves the outcome of patients with metastatic soft tissue sarcoma when compared to placebo. Sorafenib is an oral multikinase inhibitor that acts through the inhibition of Raf1 (and additional Raf isoforms) and RTK (VEGFR-1/VEGFR-2/VEGFR-3), involved both in cell proliferation and in angiogenesis [36, 37]. A multiarm phase II study by Maki et al. [38] proved an interesting activity of sorafenib in the treatment of angiosarcoma (RR 13 %; PFS 3.8; OS 14.9) and leiomyosarcoma (RR 2 %; PFS 3.2; OS 22.4), while the activity against other sarcoma subtypes was minimal. On the basis of a strong biological rational and given the encouraging results obtained in the treatment of angiosarcoma, several phase I and phase II trials are now evaluating sorafenib efficacy in the treatment of locally advanced and metastatic soft tissue sarcoma, both as a single agents and in combination with standard chemotherapy, but no phase III trials are available at the moment. Finally, given the good results obtained with trabectedin, two new compounds that act by binding DNA minor groove are under clinical development. The first one, brostallicin (PNU-166196), has been evaluated in a phase II study conducted by EORTC in metastatic or inoperable soft tissue sarcoma, and it has been found to be associated with two confirmed partial responses on 21 patients recruited and with 3 months PFS of 46 % [39]. Given the previous data, three phase II studies are currently evaluating brostallicin efficacy in advanced soft tissue sarcoma in association with doxorubicin as first-line therapy or alone as second line. Eribulin mesylate (E7389, Eisai Research Institute) is a microtubule dynamics inhibitor that is a more stable, synthetic analogue of the marine natural macrolide halichondrin B. In phase I studies, eribulin has shown a manageable tolerability profile, and a phase II study is currently evaluating its impact on 12 months PFS in the treatment of advanced soft tissue sarcoma (Table 31.3).