© Springer International Publishing Switzerland 2017
Sanziana A. Roman, Julie Ann Sosa and Carmen C. Solórzano (eds.)Management of Thyroid Nodules and Differentiated Thyroid Cancer10.1007/978-3-319-43618-0_2222. Surveillance of Treated Thyroid Cancer Patients and Thyroid Hormone Replacement and Suppression
(1)
Division of Endocrinology, Duke University Health System, Durham, NC 27710, USA
Keywords
TSH suppressionThyroid cancer surveillanceLong-term surveillance thyroid cancerTSH suppression morbidityTSH suppression goalsThyroglobulinEpidemiology
Differentiated thyroid cancer is increasing in incidence. The Surveillance, Epidemiology, and End Results (SEER) program database estimates that there will be 62,450 new cases of thyroid cancer in 2015, representing 3.8 % of all new cancers, and there will be 1950 deaths resulting from thyroid cancer. The incidence has been rising at about 5 % per year [1]. Thyroid cancer affects women more than men resulting in 47,230 of the 62,450 estimated cases to be in women. Deaths estimated in 2015 will occur in 1080 women and 870 men [2]. The yearly incidence has nearly tripled from 4.9 per 100,000 in 1979 to 14.3 per 100,000 in 2009 [3]. Nearly two out of three thyroid cancers will be detected in patients under 55 [3]. Overall, the 5-year survival for differentiated thyroid cancer is 97.9 % [1]. Many investigators feel the rise in incidence is due to detection earlier of small thyroid cancers with radiologic intensity; however, some studies have shown a rise in larger tumors being diagnosed as well [4]. Several authors including Vigneri et al. feel that thyroid cancer incidence is increasing due to two processes: (1) increased detection and (2) increased incidence due to thyroid-specific carcinogens that are not fully recognized nor studied; this latter point is the focus of ongoing investigations [5]. Almost the entire increase in incidence can be attributed to papillary thyroid cancers. Additionally, 25 % of the new thyroid cancers diagnosed in 1988–1989 were <1 cm compared to 39 % in 2008–2009 [3].
Approximately 88 % of all differentiated thyroid cancers (DTC) are papillary thyroid cancer (PTC) (and its various subtypes), while 8 % are follicular thyroid cancer [6]. DTC can occur at any age, but has a median age of diagnosis of 49 years, with approximately 39 % of new cases diagnosed prior to the age of 45 years. Women are about three times more likely to develop DTC [1]. By the year 2019, one study predicts that papillary thyroid cancer will be the third most common cancer in women at a healthcare cost of $19–21 billion in the United States alone, representing a major growing healthcare concern [7].
Prognosis
Overall prognosis for DTC is quite good. The goals of initial therapy (specifically surgery) are to (1) remove the primary tumor and any disease that has extended beyond the thyroid capsule including nodal metastases that are clinically significant; (2) minimize the risk of disease recurrence; (3) facilitate radioactive iodine ablation, adjuvant, or therapeutic, when appropriate; (4) permit accurate staging and risk stratification of the disease and prognostication; (5) permit accurate long-term surveillance for disease recurrence; and (6) minimize treatment-related morbidity [8].
In patients who have PTC tumors >1 cm with no extrathyroidal extension or vascular invasion, their risk of death is nearly 0 % and risk of recurrence on average is 2 % [9]. Patients with clinical N1 disease will have an overall risk of recurrence of 13–42 %, whereas those with pathological N1 disease will have a 7–14 % risk of recurrence [10]. Those with larger metastases and extra-nodal extension are at the highest risk for recurrence [10]. In terms of survival, stage I and II patients have a near 96–99.7 % relative 5-year survival rate vs. 91 % for stage III and 50 % for stage IV [11, 12].
Surveillance of DTC
Although most patients with DTC have a favorable long-term survival, up to 30 % of patients may experience a recurrence overall, but this is very dependent on initial staging and clinical findings [13]. Clinically evident disease recurrence has been reported up to 30 or 40 years after initial therapy, but large retrospective studies consistently show that the vast majority of recurrences are detected within 10–15 years after initial therapy [14].
Short-term and long-term surveillance strategies continue to evolve based on ongoing scientific investigations. Typically, current surveillance regimens for most patients with DTC include serial thyroglobulin measurements coupled with cervical ultrasound at a minimum to identify residual or recurrent disease, which commonly occurs within the thyroidectomy bed or lateral cervical lymph node chains [15]. In order to recommend how best to provide surveillance for a patient with treated thyroid cancer in the postoperative setting, it is important to use all of the available clinical data to individually risk-stratify patients [12]. This would include the original pathology report; pre- or postoperative neck imaging, which is typically in the form of an ultrasound; and postoperative serum thyroglobulin levels. A careful analysis of these data points can provide initial estimates for risk of recurrence, risk of having persistent disease, disease-specific mortality, and TSH suppression goals; it can guide providers in choosing the best imaging modalities for surveillance [16]. Tailoring a risk-stratified approach to individual patient care could lead to a more cost-effective approach, and possibly higher quality of life by reducing the burden of adverse treatment effects, and the stress and costs of ongoing surveillance. Providers are encouraged to stage patients postoperatively to provide prognostic information that is of value when considering disease surveillance and therapeutic strategies. In addition, this allows tracking of patients for communication among other healthcare professionals, tracking by various cancer registries and for research purposes [8].
The first-line therapy in nearly all patients is surgery, followed by radioactive iodine in some intermediate- and high-risk patients. The ATA has developed risk categories to help guide clinicians in initial treatment and subsequent surveillance as defined below.
ATA low-risk patients include papillary thyroid cancer with all of the following:
No local or distant metastases.
All macroscopic tumor has been resected.
No invasion into locoregional tissues.
Tumor does not have aggressive histology including tall cell, insular, columnar cell, Hurthle cell, or follicular cell thyroid carcinoma (FTC).
No vascular invasion.
No I131 uptake outside the thyroid bed if an I123 or I131 scan is done.
Clinical N0 or ≤5 pathological N1 micrometastases (<0.2 cm in largest dimension).
Intrathyroidal, encapsulated follicular variant of PTC.
Intrathyroidal, well-differentiated FTC with capsular invasion and no or minimal (<4 foci), vascular invasion.
Intrathyroidal, papillary microcarcinoma (<1 cm), unifocal, or multifocal, including V600E BRAF mutated if known.
ATA intermediate-risk patients include:
Microscopic invasion into the perithyroidal soft tissues (minimal extrathyroidal extension [ETE])
Cervical lymph node metastases or I131 uptake outside the thyroid bed on posttreatment scan done after thyroid remnant ablation
Tumor with aggressive histology or vascular invasion (e.g., tall cell, insular, columnar, Hurthle cell, hobnail, or FTC)
Papillary thyroid cancer with vascular invasion
Clinical N1 or >5 pathological N1 with all involved LNs <3 cm in largest dimension
Intrathyroidal, PTC with primary tumor 1–4 cm, and V600E BRAF mutated if known
Multifocal papillary microcarcinoma with extrathyroidal extension and BRAF 600E mutated (if known)
ATA high–risk patients include:
Macroscopic tumor invasion into the perithyroidal soft tissues (gross ETE)
Gross residual tumor
Distant metastases
Postoperative serum thyroglobulin suggestive of distant metastases
Pathologic N1 with any metastatic LN ≥ 3 in largest dimension
FTC with extensive vascular invasion (>4 foci)
[Adapted from the new American Thyroid Association guidelines, reference [8]]
Although most DTCs have a very favorable long-term prognosis, the disease can recur many years after initial diagnosis leading the provider to decide on a long-term plan for how best to provide surveillance for these treated thyroid cancer patients. No evidence of disease (NED) is defined as stimulated thyroglobulin <1 ng/ml with no other radiological or clinical evidence of disease. Studies looking at estimates of patients in each risk category who subsequently were characterized as no evidence of disease (NED) after total thyroidectomy and RAI remnant ablation found that 78–91 % of low-risk patients were NED; intermediate-risk patients, 52–64 % NED; and high risk, 31–32 % NED [8, 17–20]. Over a follow-up period of 5–10 years, structural disease recurrence was found in less than 1–2 % of ATA low-risk patients and 8 % of intermediate-risk patients who underwent thyroid surgery without RAI ablation as initial therapy [21–23].
It is also important to consider the clinical significance of “persistent disease.” In ATA low-risk patients, 70–80 % of persistent disease is manifest by abnormal serum thyroglobulin levels (suppressed or stimulated thyroglobulin >1 ng/ml) without identifiable structural disease, whereas in intermediate-risk patients, this ranges from 29 to 51 % and in high-risk patients 19–21 % [17, 20]. When counseling patients on risk of recurrence and tailoring surveillance strategies, the original pathology provides critical data. For example, patients with unifocal intrathyroidal papillary microcarcinomas experience structural disease recurrence of 1–2 % [24, 25] which increases to 5–6 % in 2–4 cm intrathyroidal PTCs [26] and 8–10 % in intrathyroidal PTCs >4 cm [26]. Intermediate-risk patients with locoregional lymph node involvement can have a risk of structural disease recurrence of 4 % in patients with fewer than five metastatic lymph nodes, 5 % if all lymph nodes involved are <0.2 cm, 19 % if more than five lymph nodes are involved, 21 % if >10 lymph nodes, 22 % if macroscopic lymph nodes are clinically evident (CN1 disease), and 27–32 % if any metastatic nodes are greater than 3 cm [10, 27].
Ultimately, all imaging (including structural and functional), biochemical, and cytopathological data should be used for a dynamic, ongoing redefinition of clinical status to assess the individual response to therapy at each follow-up visit over time. This re-evaluation should direct intensity of surveillance and follow-up.
Surveillance of Treated Thyroid Cancer in the First Year Post Initial Therapy
The intensity of surveillance should depend on the original risk stratification of the patient. We now have a better understanding that risk stratification is ongoing at each visit, and dynamic. As patients move through the first year and beyond following initial therapy, providers need to undertake dynamic risk stratification based on available data and use this to re-evaluate their management plans. Our newest guidelines recommend putting patients into categories of “excellent response, biochemical incomplete, structural incomplete, and indeterminate”.
Excellent response implies negative imaging, and either undetectable suppressed thyroglobulin or TSH-stimulated thyroglobulin <1 ng/ml. Patients who achieve an excellent response will have a 1–4 % risk of recurrence and a <1 % disease-specific death risk [8]. If patients are able to achieve this, they typically can undergo a decrease in intensity and frequency of follow-up and the degree of TSH suppression [8].
Patients who experience a biochemical incomplete response are characterized by negative imaging and a suppressed thyroglobulin >1 ng/ml, or a stimulated thyroglobulin >10 ng/ml, or a rising thyroglobulin antibody level. At least 30 % of these patients will spontaneously evolve to NED, 20 % will achieve NED after additional therapy, 20 % develop structural disease, and <1 % will experience disease-specific death [8]. Patients in this category who have stable or declining serum thyroglobulin levels should undergo continued observation with ongoing TSH suppression in most patients. Rising thyroglobulin or thyroglobulin antibody values should prompt additional investigations and potentially additional therapies [8].
Patients who fall into the category of structurally incomplete response will exhibit structural or functional evidence of disease with any thyroglobulin level +/− thyroglobulin antibodies. Patients in this category will continue to have persistent disease, despite additional therapy, 50–85 % of the time. The disease-specific death rate may be as high as 11 % with locoregional metastases, and 50 % with structural distant metastases. Patients with structural incomplete response may undergo additional treatments, or ongoing observation depending on multiple clinicopathologic factors including size, locations, rate of growth, RAI avidity, PET avidity, and specific pathology of the structural disease [8].
Indeterminate response includes patients with nonspecific findings on imaging studies or faint uptake in thyroid bed on RAI scanning; detectable non-stimulated thyroglobulin, but less than 1 ng/ml; detectable stimulated thyroglobulin but less than 10 ng/ml; or stable or declining thyroglobulin antibodies in the absence of structural of functional disease. Of these patients, 15–20 % will have structural disease identified during follow-up. In the remainder, the nonspecific changes are either stable or resolve. Less than 1 % will experience disease-specific death. Patients in this category should undergo continued observation with appropriate serial imaging of the nonspecific lesions and serum thyroglobulin monitoring. Nonspecific findings that become suspicious over time should be evaluated further with additional imaging or undergo therapy [8].
Thyroglobulin and cervical neck ultrasound are cornerstones of surveillance. Measurement of serum thyroglobulin is an important modality to monitor patients for persistent or recurrent disease. Following initial therapy, patients at low risk of recurrence and death can be followed with a suppressed thyroglobulin every 6–9 months in the first 2 years with no need to obtain a stimulated thyroglobulin value if there are no other suspicious clinical concerns [16]. These patients should undergo at least one follow-up neck ultrasound [16] keeping in mind that surveillance ultrasounds in this low-risk population are more likely to have false positives and lead to more procedures, including follow-up ultrasounds, FNA, and more patient anxiety.
Long-Term Surveillance of Treated Thyroid Cancer Patients
Long-term surveillance strategies also need to be individualized based on original risk of recurrence and mortality of the thyroid cancer patient. Determining accurate surveillance for possible recurrence in patients presumed disease-free is the major goal of long-term follow-up. Highly specific tests allow recognition of patients unlikely to experience disease recurrence so that less aggressive, more cost-effective, and safe management strategies can be deployed. Patients with higher risks of recurrence should be monitored more aggressively since early detection of recurrent disease is thought to offer the best opportunity for most effective care.
Most recurrences of DTC occur within the first 5–8 years after initial treatment; however, recurrences may occur even decades later, particularly in patients with PTC [28]. Long-term follow-up is guided by the evaluation of how the patient responded to therapy in the first 1–2 years of original diagnosis [12]. At each subsequent visit, patients should be classified as having one of the following clinical outcomes to direct long-term surveillance [12, 17]:
Excellent response: no clinical or biochemical or structural evidence of disease
Biochemical incomplete response: abnormal thyroglobulin levels in the absence of localized disease
Structural incomplete response: persistent or newly identified locoregional or distant metastases
Indeterminate response: nonspecific biochemical or structural findings that cannot be classified as either benign or malignant confidently
When defining an excellent response or a biochemical incomplete response, the extent of the initial therapy is key. In patients who have undergone total thyroidectomy and RAI remnant ablation, an excellent response is defined as a stimulated thyroglobulin value of <1 ng/ml or a highly sensitive non-stimulated thyroglobulin of <0.2 ng/ml with negative imaging and commonly a normal postoperative neck ultrasound. In patients who underwent total thyroidectomy without subsequent RAI therapy, a non-stimulated thyroglobulin value of <1 ng/ml is considered an excellent response. In patients treated with less than total thyroidectomy, non-stimulated thyroglobulin values less than 20 ng/ml are considered an excellent response. This equals about 50 % of the thyroglobulin expected from a normal thyroid. Any thyroglobulin value above these ranges is considered biochemical incomplete response in the absence of confirmed structural disease.
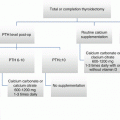
Patients classified as having an excellent response to therapy should have a decrease in the intensity of their surveillance and frequency of follow-up. They should have their TSH goal raised to 0.5–2 mU/L and be seen for physical exam and non-stimulated thyroglobulin levels yearly with surveillance neck ultrasound at 3–5-year intervals. Patients originally classified at ATA intermediate or high risk who then achieve an excellent response to therapy may benefit from closer follow-up and more intense suppression for a few more years.
Patients who demonstrate a biochemical incomplete response to therapy defined as an abnormal thyroglobulin in the absence of structurally identifiable disease should continue to be monitored every 6 months with ongoing TSH suppression and yearly neck ultrasound for several more years. Patients with stable or declining thyroglobulin values should continue routine surveillance and TSH suppression, while those with rising thyroglobulin should prompt additional imaging modalities and evaluation.
Patients deemed to have a structural incomplete response to therapy could require additional imaging or therapy depending on several clinical factors including location, rate of growth, FDG or RAI avidity, and pathology.
Patients with an indeterminate response to therapy defined as a nonspecific, biochemical, or structural imaging should continue on mild TSH suppression (0.1–0.5 mU/L) with 6-month follow-up visits for 2–3 years with yearly neck ultrasound. After that time, most patients can be reclassified [12, 17]. In summary, in terms of clinical decision making and algorithms for those who underwent partial thyroidectomy and are ATA low risk, follow-up should consist of possibly serum thyroglobulin levels and ultrasound; radioactive iodine scanning is not indicated. The TSH goal is 0.5–2 mU/L in most cases [8]. If there is an excellent response to therapy, non-stimulated thyroglobulin levels can be followed every 12–24 months with periodic neck ultrasounds [8].
In patients who received total thyroidectomy and are ATA low risk, we recommend routine use of postoperative serum thyroglobulin, and postoperative ultrasound can be used, with consideration of radioactive iodine scanning. Radioactive iodine treatment is typically not given, but if it is, low-dose ablation such as 30 mCi can be used for initial therapy [8]. Initial TSH goal is 0.1–0.5 mU/L if thyroglobulin is >0.2 and 0.5–1 mU/L if thyroglobulin <0.2. Response to therapy is evaluated by serum thyroglobulin and ultrasound, and if excellent response is detected, TSH is allowed to be 0.5–2 mU/L [8]. Once an excellent biochemical response is demonstrated, unstimulated thyroglobulin can be measured at 12–24 month intervals with periodic neck ultrasounds [8].
In ATA intermediate-risk patients who have undergone total thyroidectomy +/− prophylactic central neck and/or lateral neck dissections, routine use of postoperative serum thyroglobulin is recommended, and postoperative RAI scanning and ultrasound are to be considered [8]. For RAI remnant ablation, lower doses such as 30 mCI is generally favored over higher doses, and for adjuvant therapy, doses up to 150 mCI are administered in the absence of distant metastases [8]. The initial TSH goal is 0.1–0.5 mU/L. The response to therapy is evaluated by thyroglobulin measurement, ultrasound, and consideration of whole-body scanning. If there is an excellent response to therapy, the TSH goal can be allowed to come up to 0.5–2 mU/L and the patient be followed by periodic non-stimulated thyroglobulin and neck ultrasound imaging [8].
In ATA high-risk patients who have undergone total thyroidectomy+/− prophylactic central neck and/or lateral neck dissections, routine use of postoperative thyroglobulin is recommended, and postoperative RAI scanning and ultrasound are to be considered [8]. RAI should be considered, and adjuvant therapies up to 150 mCi are administered in the absence of distant metastatic disease. For known structural disease, 100–200 mCi or dosimetry is generally used [8]. Initial TSH goal is <0.1 mu/L. Initial response to therapy is assessed via thyroglobulin measurement and neck ultrasound, and consideration should be given to CT/MRI and/or FDG/PET scanning as well as whole-body scanning [8]. If there is an excellent response to therapy, the TSH goal should then become 0.1–0.5 mU/L for at least 5 years with yearly follow-up of thyroglobulin for 5 years and consideration of ultrasound +/−CT/MRI. If there is a biochemical or structural incomplete response, the TSH goal should be <0.1 indefinitely in the absence of contraindications [8].
Long-term survivorship care is becoming more recognized as an important area that requires future research. The American Cancer Society estimates that over 63,000 thyroid cancers were diagnosed in 2014, but there were only 1900 deaths [29]. There are over 50,000 thyroid cancer survivors alone in the United States [30]. Despite this, there remains a small amount of peer-reviewed literature on survivorship care.
Thyroglobulin in Patients With and Without RAI Treatment
Thyroid cells are assumed to the be the only source of thyroglobulin in the human body, and hence circulating thyroglobulin levels serve as a biochemical marker of persistent or recurrent disease in DTC follow-up [31]. Thyroglobulin is a large glycoprotein that in normal thyroid tissue is found in the follicular colloid where it serves as a substrate for thyroid hormone synthesis. Since it is only produced by normal or well-differentiated malignant thyrocytes, it serves as a suitable tumor marker [32]. Thyroglobulin assays became available in the 1980s and have greatly improved in sensitivity and precision [33], and have become a cornerstone in surveillance of patients post initial treatment. Since thyroglobulin has a half-life of 65 h, the levels typically nadir 4–6 weeks post-surgery [33].
The Presence of Thyroglobulin Antibodies and Challenges with Interpreting Thyroglobulin Levels
Most thyroglobulin assays are immunometric, but unfortunately are prone to interference from autoantibodies to thyroglobulin, which can occur in approximately 25 % of thyroid cancer patients and 10 % of the general population, particularly in patients with Hashimoto’s thyroiditis [34, 35].
The presence of thyroglobulin antibodies may cause a falsely low serum thyroglobulin measurement [36]. Given this, it is recommended to measure concomitant serum thyroglobulin antibodies when measuring serum thyroglobulin. No method reliably eliminates thyroglobulin antibody interference, but radioimmunoassays (RIA) for thyroglobulin may be less prone to antibody interference [37–39]. RIA assays, however, are often not as sensitive (lower limit of detection) compared to immunometric assays. Recurrent or progressive disease should be suspected in patients with rising positive antithyroglobulin antibodies, while falling levels may indicate successful therapy [40, 41]. In most patients who have undergone total thyroidectomy and RAI remnant ablation, thyroglobulin antibodies tend to disappear over a median of 3 years in patients without recurrent or persistent disease [42–44]. Several studies have shown an increased risk of recurrence or persistent disease associated with either a new appearance of antithyroglobulin antibodies or a rising titer, and thus should prompt further investigation [40, 42, 45, 46].
Imaging Modalities Used in Surveillance of DTC
Ultrasound
Ultrasound is widely used in patients with both thyroid nodules and thyroid cancer from initial detection, diagnosis, preoperative planning, and finally to postoperative surveillance. Cervical ultrasound is well suited for surveillance since most recurrences of differentiated thyroid cancer and metastases occur within the thyroid bed and in the cervical lymph node chains; it is low cost and noninvasive without radiation exposure [15]. Once a patient has had either total thyroidectomy or partial thyroidectomy, ultrasonography can be used to monitor the thyroid bed for recurrence or for evaluating for suspicious nodules in the remaining thyroid [47]. Ultrasonography can also evaluate for abnormal appearing lymph nodes in the central compartment (in a postsurgical neck) and in the lateral compartments [47].
Differentiated thyroid carcinoma, especially PTC, has been found to involve cervical lymph node metastases in 20–50 % of patients in several studies [48–50], and may be present even in the stetting of a primary tumor that is small and intrathyroidal [51, 52]. However, the clinical significance of small volume, occult lymph node metastases is still unclear.
Abnormal lymph nodes on ultrasound examination may include calcifications, cystic changes, rounded shape, hyperechogenicity, absence of a fatty hilum, abnormal vascularity, and an increased short-axis diameter [53]. Nodal microcalcifications and cystic changes are highly indicative of malignancy, and several studies have shown these two characteristics together to have reported specificities near 100 % [54]. No single sonographic feature, however, is adequately sensitive to identify malignant cervical lymph nodes with thyroid cancer.
Normal thyroid remnant tissue appears as vascular lobules of tissue with the same echogenicity of surrounding tissue. Once patients have undergone radioablation, thyroid remnants may appear as hypoechoic, heterogeneous nodules, without internal vascularity [55]. On the other hand, thyroid bed malignant recurrences typically appear as well-defined hypoechoic oval nodules. Sometimes vascularity and microcalcifications can be seen [56]. Since these features are not specific, many entities need to be considered in the differential diagnosis for recurrence, including remnant thyroid, fibrosis, suture granulomas, reactive lymph nodes, and fat necrosis [56].
If an abnormal lymph node or soft tissue is appreciated on ultrasound, confirmation of malignancy with FNA for cytology and/or measurement of thyroglobulin in the needle washout is recommended, particularly if surgical intervention will be recommended [57].
Nuclear Medicine Imaging: I123 vs. I131
Nuclear medicine imaging was once the mainstay imaging modality in the surveillance of thyroid cancer but has largely been replaced by cervical ultrasound as the primary imaging modality. Some studies have reported that whole-body scintigraphy (WBS) has a sensitivity for detection of local recurrence of 20 % vs. cervical ultrasound at 70 % [58]. Routine use of diagnostic WBS for surveillance is not recommended for low-risk patients who did not show uptake outside of the thyroidectomy bed on their initial posttreatment WBS. We still employ diagnostic WBS in patients with intermediate or high risk of recurrence. Additionally, patients with elevated or rising thyroglobulin levels with negative cervical ultrasound should also undergo WBS to assess for recurrence of radioiodine-avid disease [15].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree