INTRODUCTION
The Achilles tendon has been implicated as a major deforming force for foot pathology encountered in the diabetic patient. Specifically, a limitation of ankle joint dorsiflexion because of a contracture of the Achilles tendon is thought to be the cause. This limitation is termed equinus and has received much attention from clinicians treating diabetic foot issues, including chronic ulcerations and Charcot joint destruction. In this chapter, the relevant anatomy of the Achilles tendon, assessment of equinus, surgical techniques in the treatment for equinus, and final thoughts regarding equinus and the diabetic foot are discussed.
SURGICAL ANATOMY OF THE ACHILLES TENDON AND RELATED STRUCTURES
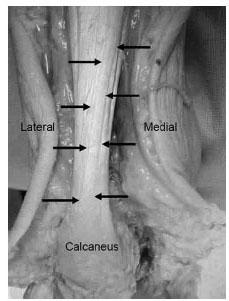
To better understand the influence of the Achilles tendon on the rest of the foot, its anatomic relationships and function must be examined. The soleus muscle originates on the posterior aspect of the tibia and fibula. The gastrocnemius muscle originates on the posterior aspect of the distal femoral condyles. The muscle bellies are supported by connective tissue called an aponeurosis, whose fibers continue distally and combine to form the Achilles tendon. The Achilles tendon inserts in unison on the posterior aspect of the calcaneus and functions to plantarflex at the ankle joint and also supinate at the subtalar joint during gait (Fig. 16.1). There is a visible rotation or torsion (from posterior to anterior) of the Achilles tendon as it progresses proximal to distal (1–4). An anatomical study conducted by Van Gils et al. demonstrated an average of 37 degrees of external rotation or lateral torsion starting about 12 cm proximal to the attachment over a tendon length of roughly 17 cm (3). This torsion should be considered when conducting an Achilles tendon lengthening. The tendinous portion of the Achilles tendon is enveloped by a tendon sheath, which allows for the tendon to slide within a closed envelope of tissue. The paratenon lies deep to the tendon sheath and is composed of fat and synovium. It functions to directly protect and nourish the tendon substance via small vessels that traverse through this layer.
The blood supply to tendons in the lower extremity is not as robust as compared with other soft tissue structures. Like all other tendons, the Achilles tendon receives its blood supply from three primary sources: the musculotendinous junction (namely, the posterior tibial artery), paratenon, and insertional location on the bone (via calcaneal periosteum and the calcaneus) (5). Studies have demonstrated that there is no completely avascular portion of the Achilles tendon. However, there is a diminished vascular segment 4 to 5 cm proximal to the insertion site on the calcaneus (6).
ASSESSMENT OF THE EQUINUS DEFORMITY
Equinus can be defined generally as a decrease in range of motion at the ankle joint due to a shortening or contracture of the posterior muscle group, including the gastrocnemius and/or soleus muscle complexes. Equinus can be determined by the inability of the foot to passively dorsiflex beyond ankle joint neutral position, although there is no clear consensus on the absolute definition of equinus. The assessment for equinus is conducted by passively dorsiflexing the foot at the ankle joint, while the subtalar joint is maintained in neutral position with the midtarsal joint maximally pronated. The traditional method for quantifying equinus is through the use of a goniometer or tractograph, with the lateral malleoli serving as the rotational axis point (7). The classic Root definition of equinus is based on the minimal range of ankle joint dorsiflexion that is necessary for normal locomotion, which Root states is 10 degrees (8). The exact amount of dorsiflexion required to be within“normal limits”is without consensus. A review of the literature of asymptomatic subjects evaluated for available ankle joint dorsiflexion reveals a range of normal numbers. This range is from 0 to 13.1 degrees in dorsiflexion with the knee extended, and 5 to 22.3 degrees with the knee flexed (9–12). Further, the weight-bearing measurement and the non–weight-bearing measurement for ankle joint dorsiflexion differ significantly. Baggett and Young reported that the non–weight-bearing measurement with knee extended is 8.25 degrees, whereas the weight-bearing measurement is 20.90 degrees (9). However, others report a decrease in dorsiflexory range of motion at the ankle joint while weight-bearing versus non–weight-bearing (11). Clearly, standardization of normal values has been difficult to establish.
Figure 16.1 A photograph of an Achilles tendon of a lower left extremity of cadaver. Note the arrows as they trace the torsion of the fibers of the Achilles tendon as it progresses from proximal medial to distal lateral at its insertion on the calcaneus.
Inconsistent technique in assessment may factor largely in misidentifying equinus. Researchers have attempted to standardize the measurement of equinus through the development of“equinometers” (13). These devices are proported to provide consistency of measurements and eliminate recorder error. DiGiovanni et al. in 2001 compared the agreement between the standard clinical assessment of equinus versus the equinometer (14). The authors argue that there is a difference in clinical measurement versus those taken from an equinometer which is more precise and reproducible. Although it would appear that the equinometer may be more precise, the use of an equinometer is impractical in a busy, nonacademic environment.
Equinus can be subdivided into gastrocnemius equinus or gastroc-soleal equinus. This subdivision can be clinically differentiated by conducting the Silfverskiöld exam (15). This exam is conducted with the knee fully extended and the knee flexed. If the foot is unable to be passively dorsiflexed beyond neutral with the knee fully extended and the knee flexed, the patient displays gastroc-soleal equinus. If the foot is unable to be passively dorsiflexed beyond neutral with knee fully extended, but is able to be dorsiflexed with the knee flexed, the patient is said to display gastrocnemius equinus (Fig. 16.2). The differentiation between gastroc-soleal equinus and gastrocnemius equinus can be explained anatomically by the fact that the gastrocnemius muscle bellies originate proximal to the knee joint. Hence, by flexing the knee the gastrocnemius is no longer under tension, which eliminates or reduces its influence. Delp et al. demonstrated this phenomenon in a computer simulation model (16). This study appears to indicate that the gastrocnemius and soleus can be individually isolated. However, others report that this delineation may not be as important to identify (17). The authors believe that this subdivision of equinus becomes important when considering various surgical lengthening procedures described in the following paragraphs.
THE LINK BETWEEN EQUINUS AND THE DIABETIC FOOT
Equinus has been cited as an important deforming force in the development and chronicity of pathologies encountered in the diabetic foot. The prevalence of equinus in the diabetic population has been reported to be 10.3% (18). Generalized limitation of joint mobility has been reported in the diabetic population (19). Specifically, the Achilles tendon undergoes structural changes such as overall thickening and increased packing density of collagen fibrils with general structural disorganization (20,21). This structural disorganization results in part from glycation-induced collagen cross-linking, which causes an increase in stiffness (22). Grant et al. further reported that the Achilles tendon of Charcot neuropathic patients exhibited decreased ultimate tensile strength and elasticity as compared with non-Charcot controls (23). These structural changes may lead to the development of an equinus deformity, which has been implicated as the cause for forefoot ulcerations in the diabetic population (24–26).
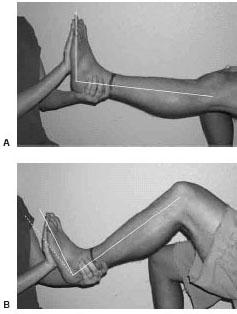
Figure 16.2 The Silfverskiöld exam to test for equinus. A. Exam conducted with the knee fully extended. Note that the foot is slightly 90 degrees to the leg. B. Exam conducted with knee flexed. The dotted line depicts previous position with the knee extended. Note that the foot has now dorsiflexed to a position to greater than neutral. This is considered gastrocnemius equinus.
Clinically, the result of these intrinsic structural changes that occur in the Achilles tendon can be measured through observing peak plantar pressures. Peak plantar pressures refer to areas on the plantar aspect of the foot that experience focalized pressures. The concept of peak plantar pressures and its relationship to diabetic feet has been examined by numerous authors. Boulton et al. report that patients with neuropathy have abnormally high pressures underneath the metatarsal heads (27). Furthermore, it appears that there is a change in load distribution on the plantar aspects of diabetic feet (28,29). Those patients with abnormally high peak plantar pressures experienced these pressures specifically at ulcer sites (Fig. 16.3). Duckworth et al. supported these findings and suggested that abnormally high peak plantar pressures play an important predictive role in the development of ulcerations (30). Others have also concluded that elevated peak plantar pressures place diabetic feet at risk for chronic ulcerations (31–33). However, the exact threshold that defines an “elevated peak plantar pressure” has not yet been clearly delineated (10,32,34).
Compounded with limited joint mobility are significant changes that occur to the soft tissue structures, including fibrotic atrophy of the plantar fat pad with thickening of the plantar fascia and the Achilles tendon (35,36). Specifically, Robertson et al. suggest that an increase in peak plantar pressures is associated with a decrease in soft tissue density on the plantar aspect of the foot, which places the diabetic foot at risk for ulceration (37). It is generally agreed that it is the combination of altered load distribution and a reduced tolerance to tissue stress that contribute to the formation and chronicity of ulcerations (38,39).
The equinus deformity appears to play a strong pathologic role in elevated peak plantar pressures, which results in a variety of diabetic foot problems. Lavery et al. directly related equinus with peak pressures by reporting that diabetic patients with equinus deformity have higher peak plantar pressures than diabetic patients that do not have equinus (18). The proposed pathologic mechanism is described by Armstrong et al. (40). This pathologic algorithm depicts an equinus deformity causing increased forces experienced in the forefoot and midfoot, resulting in plantar ulcerations and midfoot breakdown.
The majority of the Charcot collapse occurs primarily at the midfoot/Lisfranc’s area. Anatomically, midfoot breakdown accounts for roughly 60% of Charcot patients, with the forefoot being 20% and hindfoot and ankle being another 20%. The equinus forces occurring at the ankle provide a significant rotation of pressures around the ankle axis. In the neuropathic patient, the majority of increased areas of pressures appear to be along the medial column and midfoot. The result of the increased stresses located in this area result in a fairly predictable breakdown. There are several demonstrations of rotational deformities occurring at the Lisfranc’s joint from a chronic Achilles contracture demonstrated by an increase in the talar declination as well as decrease in the calcaneal inclination causing these pressure areas to persist. The longstanding deformity presents as a rocker bottom foot. This is a precursor to pressure ulcerations and more subsequent problems.
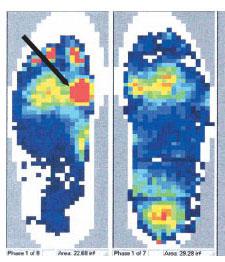
Figure 16.3 This scan depicts the sagittal plane forces experienced on the plantar aspect of the foot. The area denoted by the arrow (the left foot) indicates an area of increased focal pressure that corresponds to a chronic ulceration sub-first metatarsal head.
An equinus deformity can cause or aggravate other diabetic foot conditions as well. Biomechanically, equinus causes compensatory changes within the subtalar and midtarsal joints. This compensation creates instability, which may result in deformities such as pes planus, hallux abducto valgus, and hammertoes. These foot deformities are of significant consequence in the environment of neuropathy and ischemia, which may result in chronic ulcerations and joint breakdown. Furthermore, equinus must be addressed at the time of forefoot and midfoot amputations. The loss of the extensors creates a greater mechanical advantage by the flexors, thereby accentuating the equinus deformity. Thus, the distal stump sites are prone to breakdown and wound dehiscence because of the plantarflexed attitude of the foot. Barry et al. report a 91% healing rate after a lengthening of the Achilles tendon in the treatment of recurrent ulcerations at prior transmetatarsal amputation sites (41).
SURGICAL TREATMENT OF THE EQUINUS DEFORMITY
It is important to properly evaluate the type of equinus— gastrocnemius equinus or gastroc-soleal equinus—to ensure that the appropriate surgical lengthening is selected. In the authors’ experience, many of the patients who present for surgical reconstruction of non-Charcot and noninfective situations have been shown to have a gastrocnemius equinus. On the contrary, patients with significant Charcot deformities and chronic forefoot and midfoot ulcerations appear to more often display gastroc-soleal equinus. Clearly, there are some exceptions to both of these categories, and this should be properly evaluated and discussed with each patient. Failure to address and recognize these deformities leads to chronic recurrence of medial and forefoot ulcerations and pressures and less than optimal outcomes in the correction of any deformity. Residual equinus left in the foot and ankle will continue to cause foot breakdown even after initial wound healing or joint stabilization.
There are two fundamental surgical approaches for the reduction of the equinus deformity. As described, the Silfverskiöld test allows us to delineate between a gastrocnemius equinus versus a gastrocnemius-soleal equinus. After this determination, a gastrocnemius recession or a tendo-Achilles lengthening (TAL) should be conducted.
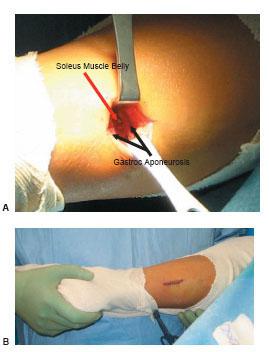
The gastrocnemius recession is a technique whereby an isolated lengthening of the gastrocnemius is conducted. A variety of techniques have been used to lengthen the gastrocnemius (Fig. 16.4) (42–45). Generally, some variation of the gastrocnemius aponeurosis is transected. This technique can be conducted either through a small open incision or endoscopically. The endoscopic technique is not discussed in any further detail here. The open technique involves the following steps (Fig. 16.5). A thigh tourniquet is not necessary for hemostasis; however, we do recommend a local block using an anesthetic solution containing dilute epinephrine. This technique can be conducted prone or with the patient in a supine position with the knee bent and the hip externally rotated. The skin incision should be made just medial to midline two to three fingerbreadths distal to the gastrocnemius muscle belly. The fatty tissue overlying the sheath of the aponeurosis should be bluntly dissected. Care must be taken to retract and protect the sural nerve that traverses this area. The aponeurosis sheath is sharply incised and freed from the underlying aponeurosis. This can easily be conducted by sweeping an index finger between the aponeurosis and overlying sheath. Now the surgeon can select the type of gastrocnemius recession that is to be conducted. The authors prefer the Strayer procedure, which involves identifying the medial and lateral borders of the gastrocnemius aponeurosis and linearly transecting this structure from medial to lateral direction using a no. 15 blade. The foot is then maximally dorsiflexed and any remaining fibers are transected. A gap should appear in the aponeurosis, exposing the underlying soleus muscle belly. If the plantaris tendon is encountered, this should be transected as well. The authors do not recommend suturing of the lengthened aponeurosis to the underlying structures.
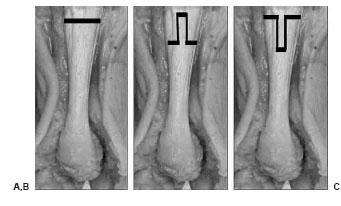
Figure 16.4 These diagrams depict the most commonly conducted gastrocnemius recessions performed. A. Strayer procedure. B. Baker distal tongue-in-groove. C. Fulp inverted tongue-in-groove.
Alternatively, a tongue and groove lengthening can be conducted. While the foot is in a maximally plantarflexed position, the distal central one-third incision or distal medial and lateral one-third incisions should be made (dependent on whether the tongue is directed proximally or distally). The foot is then maximally dorsiflexed and the proximal central one-third incision or proximal medial and lateral one-third incision should be made (again, depending on whether the tongue is directed proximally or distally). The distance between the distal and proximal incisions should be anywhere from 3 to 6 cm, depending on the size of the aponeurosis and skin incision location. Any remaining fibers are transected and absorbable or nonabsorbable sutures can be used to adjoin the lengthened aponeurosis ends. Layered closure should be conducted beginning with closing the sheath using a small absorbable suture and the subcutaneous tissue and skin with a suture of the surgeon’s preference. Postoperatively, the authors recommend non–weight-bearing with the ankle in neutral position. Full weight-bearing can begin within 2 to 3 weeks in an immobilization device.
The gastrocnemius recession has been shown to increase ankle joint dorsiflexion up to 18 degrees (46). Sharrard et al. directly compared a TAL versus a gastrocnemius recession in cerebral palsy patients with gastrocnemius equinus and gastroc-soleal equinus. They reported a lower rate of recurrence of equinus in patients who received gastrocnemius recessions (47). Furthermore, the gastrocnemius recession has certain other advantages. This approach does not cause bulbous scarring, as seen with a TAL. In fact, there is commonly a palpable delve in the area in which the recession is performed. Furthermore, there is less likelihood of Achilles tendon ruptures because the Achilles tendon is not completely compromised and there is a good blood supply to this area, which expedites healing.
The TAL is a popular method for the reduction of an equinus deformity in the diabetic patient. The modern triple hemisectioning technique for lengthening the Achilles tendon was popularized by Dr. Michael Hoke in the 1920s. In 1943, White recommended hemisectioning the medial half proximally and anterior half distally (4). Cummins recommended hemisectioning the posterior two thirds of the tendon proximally and medial two thirds distally (1). White and Cummins based their respective techniques on the anatomic torsion of the Achilles tendon. Both techniques ensured hemisectioning of both the soleal and gastrocnemius portions of the Achilles tendon. The percutaneous incisional approach to the TAL was reported by Hodgen in 1938 (48). In 1947 Hatt revisited the Hoke technique by specifically outlining the triple hemisectioning procedure (49). He described a frontal plane approach using a tenotome, in which three, one-third segments of the Achilles tendon were transected. The most proximal and distal transections were released anteriorly, whereas the middle hemisection was conducted in a posterior direction. In 1969 Conrad used a specialized instrument called a luck fasciotome to transect the medial three fourths of the Achilles tendon distally and the lateral three fourths of the tendon proximally (50). Others have reported variations in the hemisectioning of the Achilles tendon (51).
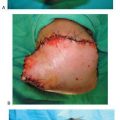
Figure 16.5 An intra-operative photograph of a Strayer gastrocnemius recession procedure.A. Dissection has been carried through the skin, subcutaneous fat, and the sheath to expose the aponeurosis. A transverse incision is conducted through the gastrocnemius aponeurosis causing the exposure of the soleus muscle belly.B. Note the location of the small incision approximately 2–3 fingerbreadths distal to the gastrocnemius muscle belly just medial to midline of the posterior leg.
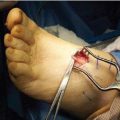
The most commonly performed TAL technique conducted today is through three percutaneous hemisections (Fig. 16.6).
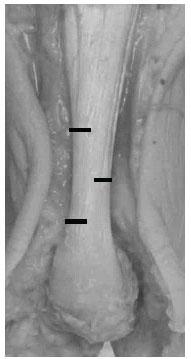
Figure 16.6 This photograph of an exposed Achilles tendon of a cadaver depicts the locations for the incisions of the lateral-medial-lateral triple hemisection technique.
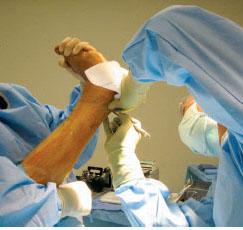
However, others advocate a complete transection of the Achilles tendon proximal to the distal attachment of the Achilles tendon on the calcaneus. Others advocate an open approach with a midline incision made over the Achilles tendon with hemisectioning or Z-plasty lengthening in the sagittal or frontal plane. The most popular TAL technique can be conducted in a prone or supine position with the leg elevated and the knee fully extended, which allows for visualization (Fig. 16.7). Prior to incision, the medial and lateral aspects of the Achilles tendon should be palpated and the skin marker should be used to make more precise cuts. The percutaneous approach involves piercing the skin, subcutaneous tissue, and Achilles tendon with a no. 11 blade in succession approximately 3 cm apart. The blade should be oriented perpendicular to the skin surface and after puncturing the tendon, the cutting edge of the blade should be rotated away from the midline. The blade should then be advanced toward the skin surface with the blade edge facing out. The proximal and distal hemisections should be made in a medial direction, whereas the central hemisection should be conducted in a lateral direction. After these incisions have been made, the foot is maximally dorsiflexed and a gap is evident where the hemisections were made. If the lengthening of the Achilles tendon does not take place, the surgical blade is reintroduced into the incision sites to attempt to release uncut fibers. The skin can be closed with a couple of sutures or can even be left to heal on its own.
Figure 16.7 This intraoperative photograph depicts a percutaneous TAL of a right leg. Note the assistant holding the lower extremity with the knee extended and the foot flexed with the patient in a supine position. The surgeon is now able to palpate the medial and lateral borders of the Achilles tendon to ensure proper hemisectioning.
The sequence of hemisectioning does not appear to matter, whether it is medial-lateral-medial or lateral-medial-lateral. The degree of lengthening does not appear to be affected, and hence is a matter of surgeon preference (52). The argument for the lateral-medial-lateral approach is based on the increased distance away from the posterior tibial artery and nerve with the distal incision. The argument for the medial-lateral-medial approach is based on the fact that the sural nerve and small saphenous vein are located laterally. The degree of increased dorsiflexion varies from 3 to 12 degrees for every centimeter of lengthening of the Achilles tendon (52,53). Non–weight-bearing status is highly individualized. Conservative management with non–weight-bearing for 3 to 4 weeks with progression to full weight-bearing may be needed. However, some surgeons allow for immediate full weight-bearing in an immobilization boot or other immobilization device. The ankle should be maintained in a neutral position. It is most likely that adjunctive procedures were conducted at the time of the TAL or gastrocne-mius recession. These adjunctive procedures are the limiting factors in the patient’s return to full weight-bearing and not the TAL or gastrocnemius recession.
CLINICAL OUTCOMES OF EQUINUS REDUCTION
The overall purpose for lengthening the gastrocnemius or the gastroc-soleal complex is to decrease the plantarflexory deforming force on the foot. There is good clinical evidence for wound healing after conducting a TAL with a healing rate for chronic diabetic ulcerations after a TAL being reported to be greater than 90% with a low reported risk of recurrence (24,25,40,54,55). Lin et al. examined diabetics with neuropathic ulcerations located on the plantar aspect of the forefoot (25). They compared a group who received a TAL and total contact casting (TCC) with patients who received TCC alone. They report no recurrence of the plantar forefoot ulcerations for the TAL group at an average of 17.3 months. The most robust study examining the efficacy of a TAL for the healing of chronic diabetic ulcerations was conducted by Mueller et al. (55). This is a prospective, randomized trial that compared a TAL plus TCC with TCC alone. They reported no difference in the number of healed ulcerations or time to healing between the two groups. However, they do report a lower recurrence rate with the TAL plus TCC group at 7 months (15%) and 2.1 years (38%) versus the TCC alone group at 7 months (59%) and 2.1 years (81%). Additionally, the recurrence of an ulcer happened sooner in the TCC alone group. Interestingly, the authors report that the peak plantar pressures and peak torque returned to baseline levels at 7 months in the TAL group despite the fact that the increase in ankle joint dorsiflexion remained at the immediate postoperative levels.
As discussed, the purpose of the TAL is to increase the range of motion at the ankle joint by lengthening the Achilles tendon, thereby reducing a deforming force in the diabetic foot. However, there are potential consequences as well. One of the more devastating complications is overlengthening the Achilles tendon. Heel ulcerations can be a result of this over-lengthening with reported rates of 13% to 14% (54,55). Hence, the patient runs the risk of potentially replacing one chronic ulceration with a potentially more devastating problem. An important addendum to this issue is raised by a study published by Shaw et al. (33). They prospectively evaluated the peak forces experienced in the foot during gait. They report that all diabetic groups displayed increased peak vertical forces as compared with healthy controls. However, these peak vertical forces occurred during heal strike, not at push off in the neuropathic diabetic group. This challenges the notion that neuropathic diabetics are at high risk of ulceration because of higher forces experienced in the plantar forefoot during gait. Furthermore, by performing a TAL these patients may be at an even higher risk of plantar heel ulcerations. Other biomechanical abnormalities may also result from overlengthening. The loss of Achilles tone may cause in compensatory flexor substitution. This can lead to a hammertoe deformity and an increase in metatarsal pressures. This in turn may lead to ulcerations located at the digits and/or submetatarsal heads.
Another potential complication of a TAL is Achilles tendon ruptures. Achilles tendon ruptures have been reported to occur 10% of the time (54). The percutaneous approach to a TAL is a blind procedure. Hence, regardless of the deliberation by the surgeon, it is possible to completely compromise the Achilles tendon. Furthermore, because of poor patient education or patient noncompliance, the surgically incised Achilles tendon is at risk for a complete rupture. This will significantly alter gait patterns and potentially cause new ulceration production in the area of the heel or contralateral limb. It is important to recall our discussion about the soft tissue changes that occur in the diabetic Achilles tendon. The Achilles tendon of a patient with diabetes already has an impaired healing response; hence, surgery may further compromise the structural integrity of the tendon (56).
CONCLUSION
An equinus deformity certainly plays a role in the development of pathologies encountered in the diabetic foot. However, the exact mechanism of how this occurs and the accurate assessment for equinus remains to be elucidated. The performance of a gastrocnemius recession or a TAL is not a one-time definitive surgery. The TAL does not address the inherent structural changes within the tendon caused by the diabetic disease process. Redevelopment of equinus should be expected, and repeated lengthening may be necessary. These repeated surgeries may have additive deleterious effects on an already-damaged Achilles tendon, further compromising this area. This may eventually lead to complete Achilles tendon rupture, causing other gait abnormalities and leading to other harmful consequences. The gastrocnemius recession and TAL continue to be a popular adjunctive procedure in the treatment of diabetic foot pathologies. There is ample evidence to support their use in this capacity; however, a TAL or gastrocnemius recession is not a panacea. The judicious use of these procedures with careful consideration for the needs of the patient must be conducted to improve overall outcomes.
REFERENCES
- Cummins EJ, Anson BJ, Carr BW, et al. The structure of the calcaneal tendon (of Achilles) in relation to orthopedic surgery. Surg Gyn Obstet 1946;83:107–116.
- Morimoto I, Ogata T. Torsion of the Achilles tendon in man and its anatomical and surgical significance. Kaibogaku Zasshi 1968;43(4):295–302.
- Van Gils CC, Steed RH, Page JC. Torsion of the human Achilles tendon. J Foot Ankle Surg 1996;35(1):41–48.
- White WJ. Torsion of the Achilles tendon: its surgical significance. Arch Surg 1943;46:784–787.
- Ahmed IM, Lagopoulos M, McConnell P, et al. Blood supply of the Achilles tendon. J Orthop Res 1998;16:5:591–596.
- Zantop T, Tillman B, Petersen W. Quantitative assessment of blood vessels of the human Achilles tendon: an immunohistochemical cadaver study. Arch Orthop Trauma Surg 2003;123:501–504.
- Nishimoto GS, Attinger CE, Cooper PS. Lengthening the Achilles tendon for the treatment of diabetic plantar forefoot ulceration. Surg Clin North Am 2003;83:707–726.
- Root ML, Orien WP, Weed JH. Normal and abnormal function of the foot. Clinical biomechanics, Vol 2. Los Angeles: Clinical Biomechanics, 1977:37–41.
- Baggett BD, Young G. Ankle joint dorsiflexion. Establishment of a normal range. J Am Pod Med Assoc 1993;83(5):251–254.
- DiGiovanni CW, Kuo R, Tejwani N, et al. Isolated gastrocnemius tightness. J Bone Joint Surg 2006;84–A(6):962–970.
- Jordan RP, Cooper M, Schuster RO. Ankle dorsiflexion at the heel-off phase of gait: a photokinegraphic study. J Am Podiatr Med Assoc 1979;69(1):40–46.
- Saxena A, Kim W. Ankle dorsiflexion in adolescent athletes. J Am Podiatr Med Assoc 2003;93(4):312–314.
- Weaver K, Price R, Czerniecki J, et al. Design and validation of an instrument package designed to increase the reliability of ankle range of motion measurements. J Rehabil Res Dev 2001;38(5):471–475.
- Digiovanni CW, Holt S, Czerniecki M, et al. Can the presence of equinus con-tracture be established by physical exam alone? J Rehabil Res Dev 2001;38(3): 335–340.
- Silfverskiöld N. Reduction of the uncrossed two-joints muscles of the leg to one-joint muscles in spastic conditions. Acta Chir Scand 1924;56:315–330.
- Delp SL, Statler K, Carroll N. Preserving plantar flexion strength after surgical treatment for contracture of the triceps surae: a computer simulation study. J Orthop Res 1995;13:96–104.
- Aronow MS, Diaz-Doran V, Sullivan RJ, et al. The effect of triceps surae con-tracture force on plantar foot pressure distribution. Foot Ankle Int 2006;27(1):43–52.
- Lavery LA, Armstrong DG, Boulton AJ. Ankle equinus deformity and its relationship to high plantar pressure in a large population with diabetes mellitus. J Am Podiatr Med Assoc 2002;92(9):479–482.
- Arkkila PET, Kantola IM, Viikari JSA. Limited joint mobility in type 1 diabetic patients: correlation to other diabetic complications. J Int Med 1994;236:215–223.
- Giacomozzi C, D’Ambrogi, Uccioli L, et al. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech 2005;20:532–539.
- Grant WP, Sullivan R, Sonenshine DE, et al. Electron microscopic investigation of the effect of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg 1997;36(4):272–278.
- Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diab Res 2004;5:143–153.
- Grant WP, Foreman EJ, Wilson AS, et al. Evaluation of Young’s modulus in Achilles tendons with diabetic neuroarthropathy. J Am Podiatr Assoc 2005;95(3): 242–246.
- Armstrong DG, Stacpoole-Shea S, Nguyen H, Harkless LB. Lengthening of the Achilles tendon in diabetic patients who are at high risk for ulceration of the foot. J Bone Joint Surg Am 1999;81A(4):535–538.
- Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-Achilles lengthening and total contact casting. Orthopedics 1996;19(5):465–475.
- Mueller MJ, Diamond JE, Delitto A, et al. Insensitivity, limited joint mobility, and plantar ulcers in patients with diabetes mellitus. Phys Ther 1989; 69(6):453–462.
- Boulton AJ, Hardisty CA, Betts RP, et al. Dynamic foot pressure and other studies as diagnostic and management aid in diabetic neuropathy. Diab Care 1983;6(1):26–33.
- Ctercteko GC, Dhanendran M, Hutton WC, Le Quesne LP. Vertical forces acting on the feet of diabetic patients with neuropathic ulceration. Br J Surg 1981;68:608–614.
- Stokes IA, Faris IB, Hutton WC. The neuropathic ulcer and loads on the foot in diabetic patients. Acta Orthop Scand 1975;46:839–847.
- Duckworth T, Boulton AJ, Betts RP, et al. Plantar pressure measurements and the prevention of ulceration in the Diabetic foot. J Bone Joint Surg 1985;67(1):79–85.
- Armstrong DG, Peters EJ, Athanasiou KA, et al. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? J Foot Ankle Surg 1998;37:303–307.
- Frykberg RG, Lavery LA, Pham H, et al. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diab Care 1998;21(10):1714–1719.
- Shaw JE, van Schie CHM, Carrington AL, et al. An analysis of dynamic forces transmitted through the foot in diabetic neuropathy. Diab Care 1998;21(11): 1955–1959.
- Lavery LA, Armstrong DG, Wunderlich RP, et al. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diab Care 2003;26(4):1069–1073.
- Brash PD, Foster J, Vennart W, et al. Magnetic resonance imaging techniques demonstrate soft tissue damage in the diabetic foot. Diab Med 1999;16(1): 55–61.
- D’Ambrogi ED, Giacomozzi C, Macellari V, et al. Abnormal foot function in diabetic patients: the altered onset of windlass mechanism. Diab Med 2005;22:1713–1719.
- Robertson DD, Mueller MJ, Smith KE, et al. Structural changes in the forefoot of individuals with diabetes and a prior plantar ulcer. J Bone Joint Surg Am 2002;84A(8):1395–1404.
- Delbridge L, Ctercteko G, Fowler C, et al. The aetiology of diabetic neuropathic ulceration of the foot. Br J Surg 1985;72:1–6.
- Lord M, Hosein R, Williams RB. Method for in-shoe shear stress measurement. J Biomed Eng 1992;14:181–186.
- Armstrong DG, Lavery LA. Elevated peak plantar pressures in patients who have Charcot arthropathy. J Bone Joint Surg Am 1998;80(3):365–369.
- Barry DC, Sabacinski KA, Habershaw GM, et al. Tendo Achilles procedure for chronic ulcerations in diabetic patients with transmetatarsal amputations. J Am Podiatr Med Assoc 1993;83(2):96–100.
- Baker LD. A rational approach to the surgical needs of the cerebral palsy patient. J Bone Joint Surg Am 1956;38A:313–323.
- Fulp MJ, McGlamry ED. Gastrocnemius tendon recession: tongue in groove procedure to lengthen gastrocnemius tendon. J Am Podiatr Med Assoc 1974;64:163–171.
- 44. Strayer LM. Recession of the gastrocnemius: an operation to relieve spastic contracture of the calf muscles. J Bone Joint Surg Am 1950;32A:671–676.
- Vulpius O, Stoffel A. Orthopadische Operationslehre. Stuttgart: Ferninand Enke, 1913:29–31.
- Pinney SJ, Hansen ST Jr, Sangeorzan BJ. The effect on ankle joint dorsiflexion of gastrocnemius recession. Foot Ankle Int 2003;24(9):726–727.
- Sharrard WJW, Bernstein S. Equinus deformity in cerebral palsy. A comparison between elongation of the tendo calcaneus and gastrocnemius recession. J Bone Joint Surg Br 1972;54(2):272–276.
- Hodgen JT, Frantz CH. Subcutaneous tenotomy of the Achilles tendon. J Bone Joint Surg Am 1938;20(2):419–423.
- Hatt RN, Lamphier TA. Triple hemisection: a simplified procedure for lengthening the Achilles tendon. N Engl J Med 1947;236(5):166–169.
- Conrad JA, Frost HM. Evaluation of subcutaneous heel-cord lengthening. Clin Orthop Relat Res 1969;64:121–127.
- Sgarlato TE, Morgan J, Shane HS, et al. Tendo Achilles lengthening and its effects on foot disorders. J Am Podiatr Assoc 1975;65(9): 849–871.
- Hoffman B, Nunley J. Achilles tendon torsion has no effect on percutaneous triple-cut tenotomy results. Foot Ankle Int 2006;27(11):960–964.
- Costa ML, Logan K, Heylings D, et al. The effect of Achilles tendon lengthening on ankle dorsiflexion: a cadaver study. Foot Ankle Int 2006;27(6): 414–417.
- Holstein P, Lohmann M, Bitsch M, et al. Achilles tendon lengthening, the panacea for plantar forefoot ulceration? Diab Metab Res Rev 2004; 20(Suppl 1):S37–S40.
- Mueller MJ, Sinacore DR, Hastings MK, et al. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am 2003;85A(8):1436–1445.
- Chbinou N, Frenette J. Insulin-dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. Am J Physiol Regul Integr Comp Physiol 2004;286:R952–R957.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree