Figure 7.4 Illustration of the four medial ankle tunnels. (a) Overview with flexor retinaculum open to show the tarsal tunnel. Note this tunnel ends at the abductor hallucis brevis (AHB). (b) The AHB has been retracted. Note the small nerve from the medial plantar nerve crossing the vessels to enter into the skin of the medial arch. This nerve must be protected. (c) The roof of the medial and of the lateral plantar tunnels has been divided. (d) The septum between the two tunnels is being divided. (e) The septum has been removed and the medial calcaneal tunnel has been decompressed. (Reproduced with permission from Dellon.com.).
The medial calcaneal tunnels are identified in one of two ways. First, there can be calcaneal nerves arising from the tibial nerve within the tarsal tunnel. These are identified in the posterior fat below the tibial nerve and are followed distally to where they enter their tunnel. Second, from the fibrous roof of the lateral plantar tunnel, that fascia is traced proximally and is found to form the roof of the calcaneal branches that arise from the lateral plantar nerve before it enters its own lateral plantar tunnel. Each of these tunnels is gently spread and then the roof carefully divided so as not to injure one of the small branches of the calcaneal nerve. None of these branches is the one described by Baxter, which is a branch arising just before the motor innervation and goes to the periosteum of the medial calcaneal tubercle.
Marcaine 0.5% is infiltrated into the skin edges. The skin is closed with multiple 4-0 Monocryl intradermal sutures. Finally, the skin is closed with interrupted and continuous 5-0 nylon sutures. The dressing is Xeroform, sterile 4 × 4 gauze, then a Kling or Kerlix gauze wrap, and finally a bulky cotton, Robert Jones type supportive dressing. This is also held on with Kling, paper tape, and finally an Ace wrap. The tourniquet is then let down. The Ace wrap is removed in half an hour and is used to just during the reflex period of vasodilation that exists after using the tourniquet (Fig. 7.6).
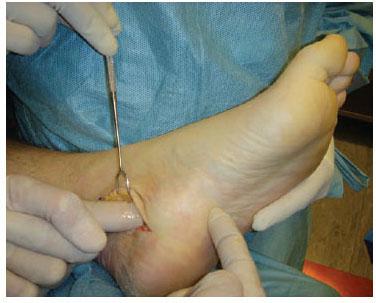
Figure 7.5 Demonstration that after decompression of the four medial ankle tunnels, the surgeon’s index finger can be passed from the incision into the plantar aspect of the foot.
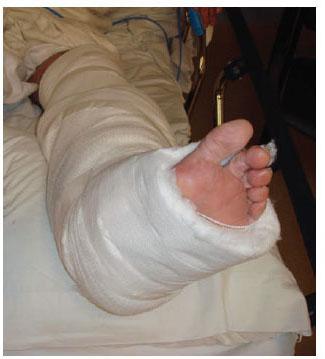
Figure 7.6 Typical postoperative bulky supportive dressing.
POSTOPERATIVE MANAGEMENT
Postoperatively, the patient is allowed full weight bearing immediately and uses a walker for 3 weeks. The goal of walking is to minimize ankle range of motion so that the sutures do not pull out, yet still permit nerve gliding. The dressing is removed after the seventh day, and the patient is allowed to get the sutures wet, but must apply Betadine twice a day to the incisions. The patient changes chairs each hour while awake to permit nerve gliding and minimize the risk of a deep vein thrombosis. The sutures at the knee are removed at the 14th day and at the foot/ankle level at the 21st day. Following removal of the sutures at the ankle, the patient is begun on water walking in a heated pool as a form of therapy. This should occur at least twice a week and preferably three times a week. Usually, no other therapy is necessary. The patient will then progress through increasing degrees of ambulation and activity as tolerated.
As pain diminishes, pain medication is decreased. In the patient who did not have preoperative pain and who experiences pain because of nerve regeneration, a regimen of neuropathic pain medication can be started and the opioids continued as needed.
Repeat neurosensory testing is done at 6 to 12 weeks postoperatively to document sensory recovery. It may be done sooner if the patient is experiencing significant pain, as the neurosensory test documents a nerve regeneration pattern that is reassuring to both the patient and the physician.
The contralateral side may be operated on as early as 6 weeks later, if there has been sufficient documentation of pain relief or sensory recovery. Typically, most patients wait about 3 months to have surgery on the contralateral side. The longest time interval has been 1 year.
TIPS TO AVOIDING COMPLICATIONS
The most common “complication” is that 10% to 20% of patients do not improve following this surgery. The most common cause for this is previous back surgery with residual symptoms in the legs from the back problem. This is extremely difficult to identify preoperatively in the presence of a neuropathy and peripheral nerve compressions. A second cause of failure to improve is another site of nerve compression. Just recently, we have focused upon entrapment of the tibial nerve proximally, at the calf level, where the tibial nerve goes beneath the soleus arch. There will be tenderness at the location and weakness in toe flexion. This site can be decompressed through a medial calf incision, going deep to the medial gastrocnemius muscle, and dividing this arch.
The second most common complication is wound healing problems. The most common site is the medial ankle incision. In about 5% of patients, this may become red or may open. The sutures create a problem with the skin with early ambulation, but such ambulation is critical to prevent the tibial nerve from becoming scarred in the surgical site. The patient is given IV antibiotics prior to inflating the tourniquet during the surgical procedure and for 1 week postoperatively. With advanced neuropathy, there is no pain at this incision site to warn the patient that the ankle is moving too much. Use of the bulky supportive bandage the first week and the walker for 3 weeks, with the patient initiating each step by lifting the knee first minimizes suture and skin trauma. In my personal experience, no wound has had to be skin grafted. Six patients over 20 years have required secondary healing. Also, be careful not to cauterize the dermis while obtaining hemostasis.
Preoperative attention to blood flow is critical to wound healing. To date, none of my patients with neuropathy has required secondary vascular surgery intervention. If a patient has had a previous bypass arterial graft, our current recommendation is not to use a tourniquet during the surgery (59,60).
An occasional patient has developed a deep vein thrombosis. We seek to prevent this by using compression stockings in the operating room on the contralateral leg, and having the patient ambulate immediately and often, albeit for short distances, during the early postoperative period. If the patient is driving >1.5 hours home after surgery, they are told to stop the car each hour and get out and then get back into the car through the opposite side of the car.
Patients must be informed that they may have a neurologic downgrade in motor or sensory function, but they are usually quite impaired prior to surgery, and this downgrading is extremely rare, happening 0.5% of the time. Prevention requires attention to the preceding surgical details and meticulous, gentle, peripheral nerve surgical skills.
EXAMPLE OF SURGERY
A 66-year-old man had a 5-year history of numbness progressing to burning pain in both of his feet. The symptoms involved each foot to about the same degree. He did not have lumbar spine problems. He was told he had a neuropathy of unknown etiology. The neuropathy was confirmed by electrodiagnostic studies. He was placed on Neurontin to help manage his level 8 out of 10 pain. He was overweight and had a family history of diabetes. His cholesterol and blood pressure were both elevated and he was on appropriate medications for each of these conditions. Three years ago, he was given the diagnosis of non–insulin–dependent diabetes mellitus, and his foot symptoms were said to be because of diabetic neuropathy. He was taking an oral medication for his diabetes and his last HbA1c was 6.8. He had begun to lose his balance and had fallen twice without having sustained a fracture. He did not have a history of ulceration in his foot.
At the time he was first examined, he was found to have a positive Tinel sign bilaterally over the CPN at the fibular neck, the DPN over the dorsum of the foot, and the tibial nerve in the tarsal tunnel, demonstrating that he did have localized sites of chronic nerve compression present in each lower extremity. He did not have a Tinel sign over the superficial peroneal nerves, nor was the tibial nerve tender behind the calf. He had mild wasting of his abductor hallucis, but he did not have clawing of his toes. He had weakness in his extensor hallucis longus muscle. He had normal strength in his toe flexors. There was a strong posterior tibialis pulse present bilaterally and no pedal edema.
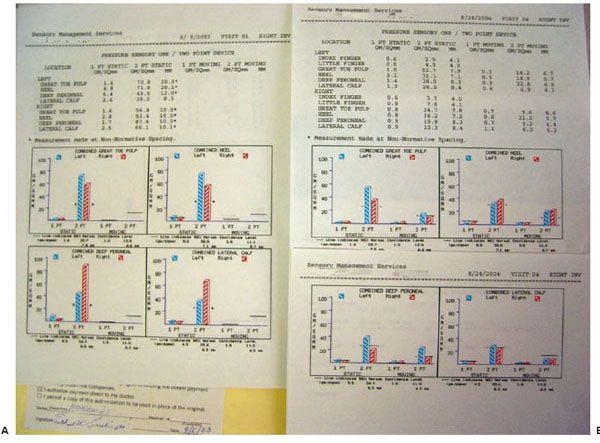
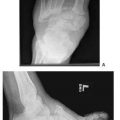
His initial neurosensory test with the pressure-specified sensory device documented a sensory neuropathy with axonal loss (Fig. 7.7A).
He was determined to be an excellent candidate for peripheral nerve decompression, and was scheduled to have a neurolysis of the CPN at the fibular neck, neurolysis of the DPN over the dorsum of the foot, and a decompression of the four medial ankle tunnels. (Because three incisions are made, this has been termed the Dellon triple.)
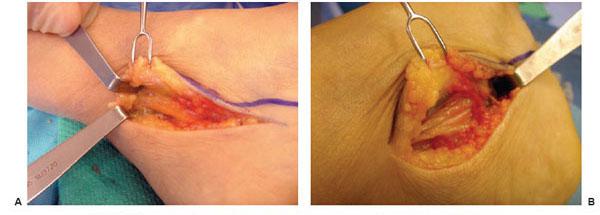
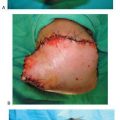
On the left foot surgery, the CPN was found to be infiltrated by fat and to be severely compressed (Fig. 7.8A–C). The tibial nerve in the tarsal tunnel also demonstrated signs of fatty infiltration and tightness in the medial and lateral plantar tunnels (Fig. 7.9A,B).
In the recovery room immediately after surgery, it felt ticklish and he laughed (a positive test tickle sign) when his left plantar skin was gently stroked (Fig. 7.10). After surgery, his pain level in the left foot fell to 4 out of 10, and by the third postoperative month, his repeat neurosensory testing demonstrated nerve regeneration in his left foot.
At 4 months after the first surgery, he had the same surgery on his right foot.
By 7 months after the first surgery, with neurosensory testing documenting recovery to almost normal levels of sensibility in both of his feet (Fig. 7.7B), he had regained his balance and had no further falls. With recovery of sensibility, his likelihood of ever developing ulceration or having an amputation (other than for vascular reasons) was greatly reduced. He was asked to return in 1 year for follow-up.
Figure 7.7 Neurosensory testing with the pressure-specified sensory device. A. Preoperative testing documents a bilateral sensory neuropathy with axonal loss. The red bar represents the right foot and the blue bar represents the left foot. The y-axis represents pressure applied for the patient to be able to distinguish the stimulus. The black horizontal lines are the 99% confidence limit for age-matched normal data. The red and blue bars are symmetrically elevated for both the peroneal and tibial nerves, consistent with neuropathy. The asterisk means that two-point discrimination distance is abnormal, consistent with axonal loss. B. Postoperative testing demonstrates regeneration of sensory axons, now to almost normal levels.
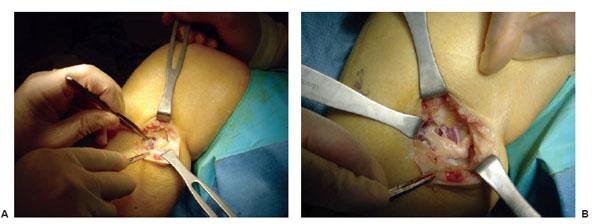
Figure 7.8 Example of surgery on the left common peroneal nerve entrapment site. A. Note the yellowish color of the common peroneal nerve as it approached the peroneus longus muscle. B. Common peroneal nerve after neurolysis. Note indentation of the nerve.
Figure 7.9 Example of surgery on the left tarsal tunnel. A. Note yellowish change in color in the tibial nerve in the tarsal tunnel. B. Within the tarsal tunnel, the tibial nerve has divided into medial and lateral plantar and calcaneal nerve. The retractor elevates the abductor hallucis, demonstrating the white roof of the medial and lateral tunnels that remains to still be divided.
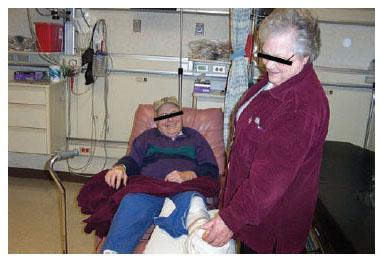
Figure 7.10 This patient in the recovery room smiles as his wife tickles his foot (a positive test tickle sign), indicating that the decompression has permitted reversible ischemia of the nerves to allow some of the sensory nerves (those with a conduction block but not degeneration) to function again. His balance will be regained after he has surgery bilaterally to decompress these nerves.
REFERENCES
- Peek ME, Cargill MA, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007;64(Suppl 5): 101S–156S.
- Piatt GA, Zgibor JC. Novel approaches to diabetes care: a population perspective. Curr Opin Endocrinol Diabetes Obes 2007;14:158–165.
- Hossain P, Kawar B, Nahas ME. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med 2007;356:213–215.
- Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1991;43:817–824.
- Latov N. Diagnosis of CIDP. Neurology 2002;59(12 Suppl 6):S2–S6.
- Dyck PJ, Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve 2007;36(4):536–541.
- Sorbe B, Andersson H, Boman K, et al. Treatment of primary advanced and recurrent endometrial carcinoma with a combination of carboplatin and paclitaxel–long-term follow-up. Int J Gynecol Cancer 2007;18:803–808.
- Feyler S, Rawstron A, Jackson G, et al. Thalidomide maintenance following high-dose therapy in multiple myeloma: a UK myeloma forum phase 2 study. Br J Haematol 2007;139:429–433.
- Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962.
- Rather HM, Boulton AJ. Recent advances in the diagnosis and management of diabetic neuropathy. J Bone Joint Surg Br 2005;87:1605–1610.
- Argoff CF, Backonja MM, Belgrade MJ, et al. Consensus guidelines: treatment planning and options. Diabetic peripheral neuropathic pain. Mayo Clinic Proc 2006;81(Suppl 4):S12–S25.
- Dyck PJ, Norell JE, Tritschler H, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care 2007;30:2619–2625.
- Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001;86:32–38.
- Ivers RQ Cumming RG, Mitchell P, et al. Diabetes and risk of fracture. Diabetes Care 2001;24:1198–1203.
- American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–932.
- Vinik AI, Mehrabyan A, Colen L, et al. Focal entrapment neuropathies in diabetes. Diabetes Care 2004;27:1783–1788.
- Boulton AJ, Malik RA, Arezzo JC, et al. Diabetic somatic neuropathies. Diabetes Care 2004;27:1458–1486.
- Dellon AL. A cause for optimism in diabetic neuropathy. Ann Plast Surg 1988;20: 103–105.
- Chaudhry V, Stevens JC, Kincaid J, et al. Practice advisory: utility of surgical decompression for treatment of diabetic neuropathy: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2006;66:1805–1808.
- Cornblath DR, Vinik A, Feldman E, et al. Commentary: surgical decompression for diabetic sensorimotor polyneuropathy. Diabetes Care 2007;30:421–422.
- Rempel D, Evanoff B, Amadio PC, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health 1998;88:1447–1451.
- Szabo RM, Slater RR, Farver TB, et al. The value of diagnostic testing in carpal tunnel syndrome. J Hand Surg [Am] 1999;24:704–714.
- AAEM Quality Assurance Committee. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve 1993;16:1392–1414.
- Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care 2002;25:565–569.
- Greenwald D, Moffitt M, Cooper B. Effective surgical treatment of cubital tunnel syndrome based on provocative clinical testing without electrodiagnostics. Plast Reconstr Surg 1999;104:215–218.
- Lee CH, Dellon AL. Prognostic ability of Tinel sign in determining outcome for decompression surgery decompression surgery in diabetic and nondiabetic neuropathy. Ann Plast Surg 2004;53:523–527.
- Aszmann OC, Tassler PL, Dellon AL. Changing the natural history of diabetic neuropathy: incidence of ulcer/amputation in the contralateral limb of patients with a unilateral nerve decompression procedure, accepted. Ann Plast Surg 2004;53:517–522.
- Ducic I, Taylor NS, Dellon AL. Relationship between peripheral nerve decompression and gain of pedal sensibility and balance in patients with peripheral neuropathy. Ann Plastic Surg 2006;56:145–150.
- Mont MA, Dellon AL, Chen F, et al. The operative treatment of peroneal nerve palsy. J Bone Joint Surg Am 1996;78:863–869.
- Dellon AL, Ebmer J, Swier P. Anatomic variations related to decompression of the common peroneal nerve at the fibular head. Ann Plast Surg 2002;48:30–34.
- Rosson GD, Dellon AL. Superficial peroneal nerve anatomic variability changes surgical technique. Clin Orthop Relat Res 2005;438:248–252.
- Williams EH, Dellon AL. Intraseptal superficial peroneal nerve. Microsurgery 2007;27:477–480.
- Dellon AL. Entrapment of the deep peroneal nerve on the dorsum of the foot. Foot Ankle 1990;11:73–80.
- Mackinnon SE, Dellon AL. Homologies between the tarsal and carpal tunnels: implications for treatment of the tarsal tunnel syndrome. Cont Orthop 1987;14:75–79.
- Dellon AL. Treatment of symptoms of diabetic neuropathy by peripheral nerve decompression. Plast Reconstr Surg 1992;89:689–697.
- Kim J, Dellon AL. Tarsal tunnel incisional pain because of neuroma of the posterior branch of saphenous nerve. J Am Podiatr Med Assn 2001;91:109–113.
- Dellon AL, Mackinnon SD. Tibial nerve branching in the tarsal tunnel. Arch Neurol 1984;41:645–646.
- Dellon AL, Kim J, Spaulding CM. Variations in the origin of the medial calcaneal nerve. J Am Podiatr Med Assoc 2002;92:97–101.
- Kim J, Dellon AL. Neuromas of the calcaneal nerves. Foot Ankle Int 2001;22:890–894.
- Wieman TJ, Patel VG. Treatment of hyperesthetic neuropathic pain in diabetics. Decompression of the tarsal tunnel. Ann Surg 1995;221:660–665.
- Chafee H. Decompression of peripheral nerves for diabetic neuropathy. Plast Reconstr Surg 2000;106:813–815.
- Aszmann OA, Kress KM, Dellon AL. Results of decompression of peripheral nerves in diabetics: a prospective, blinded study. Plast Reconstr Surg 2000;106;816–822.
- Tambwekar SR. Extended neurolysis of the posterior tibial nerve to improve sensation in diabetic neuropathic feet. Plast Reconstr Surg 2001;108:1452–1453.
- Wood WA, Wood MA. Decompression of peripheral nerves for diabetic neuropathy in the lower extremity. J Foot Ankle Surg 2003;42:268–275.
- Biddinger KR, Amend KJ. The role of surgical decompression for diabetic neuropathy. Foot Ankle Clin 2004;9:239–254.
- Valdivia JM, Dellon AL, Weinand ME, et al. Surgical treatment of peripheral neuropathy: outcomes from 100 consecutive decompressions. J Am Podiatr Med Assoc 2005;95:451–454.
- Rader AJ. Surgical decompression in lower-extremity diabetic peripheral neuropathy. J Am Podiatr Med Assoc 2005;95:446–450.
- Yao Y, Wang RZ. Peripheral nerve decompression (Dellon procedure) and diabetic neuropathy. Chin J Med 2005;10:1756–1758.
- DiNucci K. Results of decompression of multiple lower extremity peripheral nerves in diabetic with symptomatic neuropathy. Paper presented at: American College of Foot and Ankle Surgery Meeting; New Orleans, 2005.
- Steck J. Results of decompression of lower extremity peripheral nerve for treatment of symptomatic neuropathy. Paper presented at: American Society of Peripheral Nerve Meeting, Puerto Rico, 2005.
- Siemionow M, Alghoul M, Molski M, et al. Clinical outcome of peripheral nerve decompression in diabetic and nondiabetic peripheral neuropathy. Ann Plast Surg 2006;57:385–390.
- Yuksel F. Lower extremity nerve decompression in diabetic neuropathy. Paper presented at: Annual Meeting of the American Society of Peripheral Nerve, Tucson, 2006.
- Shafiroff B. Decompression of lower extremity nerves in neuropathy. Paper presented at: Annual Meeting of the Lower Extremity Peripheral Nerve Surgery, Sante Fe, 2006.
- Nelson SC, Little ER Jr. The 36-item short-form health survey for outcome evaluation for multiple lower-extremity nerve decompressions in diabetic peripheral neuropathy: a pilot study. J Amer Podiatr Med Assoc 2007;97:121–125.
- Massa E. Diabetic neuropathy: decompression of compressed lower extremity nerves. Paper presented at: Annual Meeting of the International Diabetes Federation, Cape Town, 2007.
- Maloney CT, Valdivia JV, Weinand M. Nerve decompression results in a consecutive series of 165 patients with neuropathy. Neurolog Surg, in press.
- Bae S, Biddinger K, Shon L. Independent, retrospective review of surgical nerve decompression for diabetic neuropathy. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgery Foot & Ankle Society, San Diego, 2007.
- Periera BM. Decompressive peripheral nerve surgery for the relief of neuropathic symptoms in diabetics. Paper presented at: Annual Meeting of the American Association of Neurologic Surgeons; 2007.
- Ducic I, Chang S, Dellon AL. Use of tourniquet in reconstructive surgery in patients with previous ipsilateral lower extremity revascularization: is it safe? A survey. J Reconstr Microsurg 2006;22:183–189.
- Dellon AL. Medical and surgical management of diabetic neuropathy. Clin Podiatr Med Surg 2007;24:425–428.
INTRODUCTION
Infections in the diabetic foot may lead to limb loss. Recognition of infection and the ability to manage it surgically is often a challenge. An aggressive approach to managing these cases has been shown to reduce the overall rate of major amputations. Efficient and definitive surgical intervention, combined with appropriate antibiotic therapy, is the cornerstone of limb salvage in the infected diabetic foot.
DECISION-MAKING FACTORS FOR SURGICAL INTERVENTION
The surgical approach to the infected diabetic foot is dependent on an aggressive physical exam. The physician needs to understand that surgery should be considered an integral part of the overall approach to diabetic foot infections. Upon initial examination of a diabetic foot wound, care should be taken to adequately assess for signs of infection. Although many patients present with cardinal signs of infection—erythema, warmth, swelling, and drainage—there are often those patients in whom the presentation of infection is more subtle. It is in these patients that a more aggressive examination becomes imperative to determine the need for and extent of surgical intervention. A probe passed into a wound may identify involvement of fascial planes or tendon sheaths, as well as areas of fluctuance or abscess formation that may not be appreciated on a cursive exam (Fig. 8.1). Failure to adequately identify a deep-space infection either by a limited exam, or any other delay (often while awaiting the results of diagnostic testing, such as nuclear medicine scans, CT, or MRI) may result in further tissue loss, with resultant compromise of limb salvage. An experienced surgeon understands that as little as 24 hours of undrained sepsis will determine whether the patient requires a partial foot amputation or even minimal tissue loss.
In conjunction with the patient’s clinical exam, the other potential signs of systemic infection need to be evaluated as well. However, although fevers, chills, hyperglycemia, and leukocytosis may be present, it is not uncommon to have these patients present with an absence of these signs or symptoms. Frequently, they present with just elevated blood sugar. The physical exam is most often all that is needed to determine the timing and extent of incision and drainage (I&D). In fact, the examination should not be done without a sterile I&D kit (blunt probe, scissors, forceps, and disposable scalpel) readily at hand, not only to drain an abscess, but also to aid in the aggressive physical exam (Fig. 8.2).
CLASSIFICATION OF INFECTION
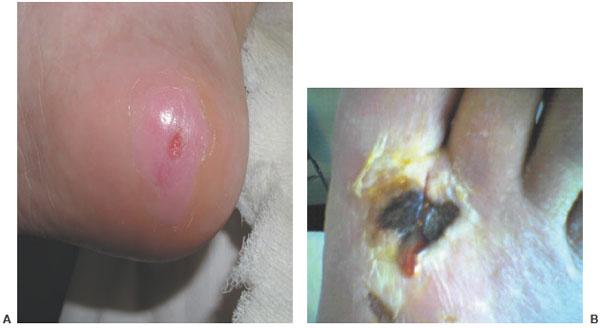
To properly guide treatment, the practitioner should understand a classification system for infection. The preferred system at our institution keeps it simple. It categorizes infections into non-limb-threatening and limb-threatening infections (Table 8.1) (1,2). Non-limb-threatening infections are superficial ulcerations that do not involve bone and do not have significant ischemia or systemic toxicity. Cellulitis does not extend beyond 2 cm around the wound. The patient is medically stable and can be treated in an outpatient setting (Fig. 8.3A,B). Surgery is usually a minor débridement with an in-office incision and drainage (Fig. 8.4A,B). Wound culture is obtained by removing a small piece of tissue as deep as possible. This is followed with an oral antibiotic, which has predominantly gram-positive coverage. X-rays are done to evaluate for osteomyelitis. The patient is then re-evaluated in 1 week. A more aggressive I&D is warranted if the patient is not responding to therapy.
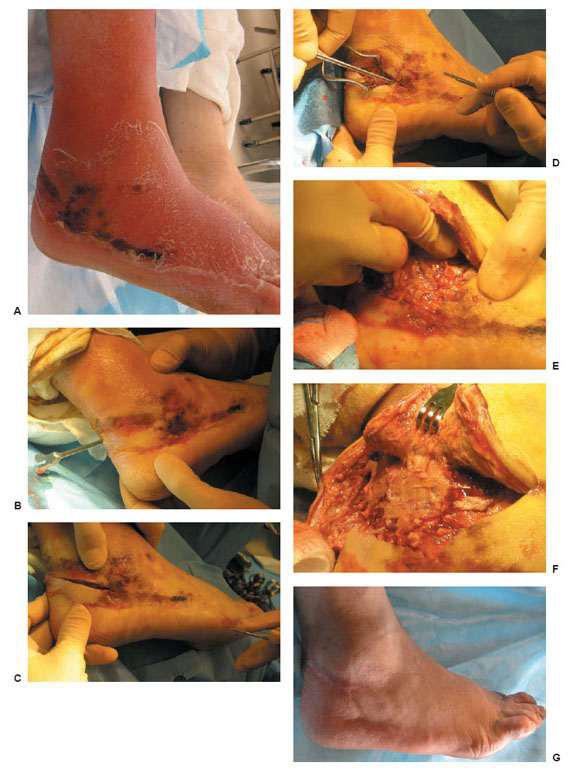
Limb-threatening infections are characterized by wounds with cellulitis of >2cm, significant swelling, abscess formation, lymphangitis, gangrene, necrotizing fascitis, and osteomyelitis (Figs. 8.5A,B and 8.6A-G). Often, these patients are medically unstable and have significant hyperglycemia or labile blood sugars. In addition to a complete history and physical, an aggressive physical exam of the entire lower extremity is most important to determine the degree of surgical intervention and need for hospitalization. Adjunct parenteral broad spectrum antibiotics are started. Empiric coverage of methicillin-resistant Staphylococcus aureus (MRSA) should also be considered. Tentolouris et al (3) in 1999 and Cook et al (4) in 2007 reported significant delay in wound healing and increased amputation rates when MRSA was the primary pathogen compared to wounds infected with methicillin-susceptible Staphylococcus aureus (MSSA). When faced with a severe limb-threatening infection, a multidisciplinary approach becomes necessary. These patients, who are at times medically unstable, still need to be taken to the operating room. Such life-threatening infections necessitate immediate surgical attention without delay (1). They should be considered for emergent incision and drainage, débridement, and if necessary, guillotine amputation. A delay in surgical decision making may manifest itself as further tissue loss, loss of limb, or even, as approach becomes necessary. These patients, who are at times medically unstable, still need to be taken to the operating room. Such life-threatening infections necessitate immediate surgical attention without delay (1). They should be considered for emergent incision and drainage, débridement, and if necessary, guillotine amputation. A delay in surgical decision making may manifest itself as further tissue loss, loss of limb, or even, as Joshi et al. in 1999 reported, loss of life in up to 40% of diabetic patients who had necrotizing fasciitis (5).
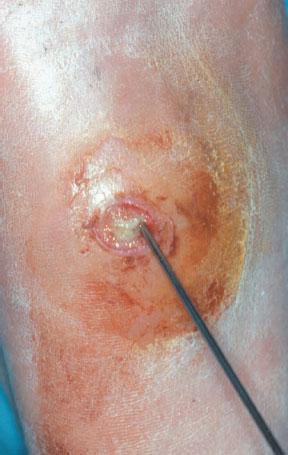
Figure 8.1 Small benign-appearing ulcer is always probed for an abscess, undermining of tissue, or extension to bone.
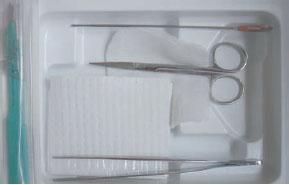
Figure 8.2 Sterile, inexpensive incision and drainage kit with disposable no. 15 blade for office and bed side use.
TABLE 8.1
Non-limb-threatening infection
Superficial: No bone or joint involvement
Minimal or no cellulitis
No significant ischemia
No systemic toxicity
Microbes: Usually gram-positive
Limb-threatening infection
Deep ulcer (± bone or joint involvement)
Superficial ulcer: If foot is ischemic
Cellulitis: 2 cm
Lymphangitis
Systemic toxicity
Microbes: Polymicrobial or methicillin-resistant Staphylococcus aureus (MRSA)
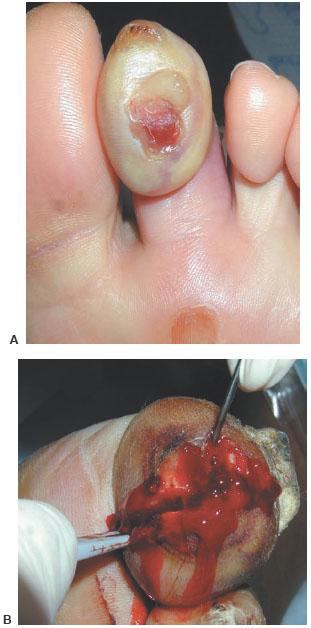
Figure 8.3 A,B. Incision and drainage without delay at the bedside.
Figure 8.4 A,B. Non–limb-threatening infections. Note incision in (B), which was done in the office during the initial examination.
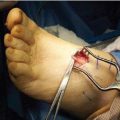
Appropriate clinical evaluation of perfusion to the foot is also necessary before aggressive incision and drainage. However, if a severe limb-threatening infection needs to go to the operating room urgently, vascular studies are done subsequent to the incision and drainage. The foot and ankle surgeon needs to fully understand the detriments of arterial insufficiency and its effect on wound healing and infection control, and must take this into account when planning incisions. In these situations, it is of the utmost importance to have an experienced foot and ankle surgeon involved with the initial evaluation and intraoperative decisions. It is preferable to make incisions in a linear fashion and completely filet open the infected area along tissue planes. A limited approach with simple stab incisions is not recommended. The authors have found that less experienced foot and ankle surgeons are hesitant to completely open all questionable areas and remove nonviable tissue (Fig. 8.7A–D). An example of this is an exposed tendon that does not yet appear necrotic. Most often, such tendons provide a nidus for recurrent infection and need to be accounted for in later reconstructive procedures. The intraoperative consideration of subsequent procedures is best done by a surgeon with extensive experience with tissue loss who can immediately plan for the wound closure. Poorly planned incisions preclude the best reconstructive flap or foot-sparing amputation. In addition, dry eschars in the face of significant arterial insufficiency do not need to be débrided if there are no signs of infection, as this predictably results in the formation of further tissue loss and another necrotic eschar.
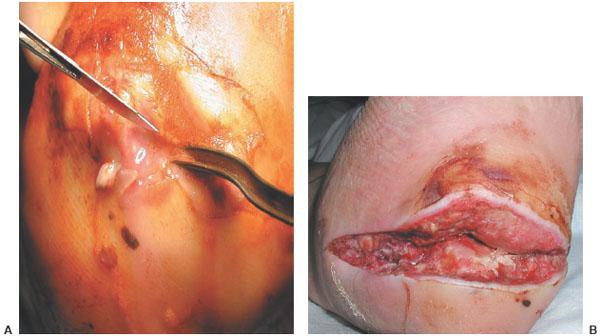
Figure 8.5 A,B. Plantar heel ulcer with deep abscess intraoperatively and 10 days postoperatively awaiting closure.
Figure 8.6 A–G. Hidden deep infection requiring aggressive blunt separation of infected tissue planes.
Figure 8.7 A–D. Complete excision of all infected tissue in a limb-threatening abscess with osteomyelitis
At our institution, diabetic foot infections are managed by the podiatric surgical and/or vascular surgical services. The aggressive, early surgical approach we employ, as opposed to conservative nonsurgical therapy, has been substantiated in a study by Tan et al (6) in 1996. This study showed that early, aggressive surgical intervention decreased major amputation rates.
OSTEOMYELITIS
In 1996, a patient with the diagnosis of diabetes carried a 15% lifetime risk of developing a foot ulcer (7,8). Diabetic foot ulcers which extend to bony structures increase the risk of osteomyelitis. The triad of diabetes, ulceration, and bone infection has increased in prevalence and are now the leading cause of nontraumatic lower-extremity amputations. Despite the tremendous advances in prosthetic limbs, the 5-year mortality rate following major amputation ranges between 39% and 80% (9,10). The urgency and consequences of this pathology are well understood; however, discordance remains with regard to the best treatment for osteomyelitis. The treatment options for osteomyelitis include treatment with antibiotics alone or in combination with surgical excision.
The conclusive diagnosis of osteomyelitis is obtained by bone biopsy. However, many times a wound culture of a soft tissue sinus tract serves as diagnostic. Although antibiotic therapy is initiated based on sinus tract cultures, the course of treatment may be less certain or require broader-spectrum antibiotics, even if the soft tissue infection resolves or improves based on sinus tract cultures. Mackowiak et al in 1978 stated that cultures obtained from draining sinus tracts and soft tissue specimens at the level of infected bone frequently fail to identify the organisms responsible for creating the osteomyelitis, except for Staphylococcus aureus or a monomicrobial infection (11). Their data showed that of 183 cultures taken from 35 patients, 102 did not coincide with operative cultures. Lavery et al in 1995 also reported that organisms isolated from soft tissue cultures were only found in 36% of 36 patients with osteomyelitis (12).
When antibiotic therapy is initiated before a bone biopsy can be obtained, the bacteria may be suppressed and therefore can interfere with accurate identification of the infecting organism. When this occurs, a Gram stain of the bone specimen may be helpful in determining the infecting organism. Currently, at the authors’ institution, the diagnostic approach to a patient with suspected osteomyelitis in a wound that probes to bone, plain film radiographs are obtained to determine the extent of osseous erosion as well as to assess anatomy for surgical planning. On rare occasions, magnetic resonance imaging is employed to differentiate osteomyelitis from a neuropathic joint. Assessment of the patient’s vascular status is paramount to the success of the surgical débridement. The presence of vascular disease is a contributing factor in the development of the infection as well as a cause for delayed healing and continued sepsis (13). A consult to an experienced vascular surgeon may be necessary to restore adequate perfusion to the foot and allow for healing.
Osteomyelitis is described as an infection of bone and marrow. Suppuration of bone cortex without marrow extension is referred to as osteitis, and contamination of periosteum alone is termed periostitis (14). Differentiation of these maladies is rarely done. The plethora of literature advocating emphasis on medical therapy, however, should require definitive identification of each. As would be expected, complete eradication without recurrence varies greatly when treating just periostitis versus frank osteomyelitis.
When the practitioner is faced with the decision to act quickly and aggressively in a diabetic patient with pedal osteomyelitis, it is more important to consider whether it is acute or chronic. Acute osteomyelitis, in addition to presenting different clinically, has definitive characteristics histologically. The pathologist describes it as an intense neutrophilic inflammatory infiltrate at the site of bacterial invasion accompanied by edema, vascular congestion, and small vessel thrombosis. In the early acute disease, the vascular supply to the bone is compromised by infection of the surrounding soft tissue and the bone becomes necrotic within a matter of days. When the medullary and periosteal blood supply is compromised, large areas of dead bone (sequestra) may be formed. When this occurs, the bacteria can be difficult to eradicate even after intense host response, surgery, and antibiotic therapy (14). The experienced clinician understands that this requires expedient ablative surgery and in many instances primary amputation.
On the other hand, although surgery eventually provides a definitive cure, chronic osteomyelitis does not require such emergent treatment. The clinician can treat the soft tissue infection with débridement and antibiotics, which converts the acute soft tissue infection into a chronic wound (Fig. 8.8A–D). This allows the option to single-stage surgical resection with flap or primary closure (15). If a large dead space exists, the area could be temporized with antibiotic impregnated beads in preparation for second-stage reconstruction. Such temporizing is evidenced by the different histologic presentation of chronic osteomyelitis. The slow insidious onset of the disease is characterized by an influx of chronic inflammatory cells into the focus of osteomyelitis, which initiates a repair reaction that includes osteoclast activation, fibroblast proliferation, and new bone formation. The residual larger sequestrum is eventually surrounded by a rim of reactive bone forming an involucrum, which is a sheath of live bone encasing dead bone. Often, the involucrum is perforated by openings, thus forming sinus tracks from which pus may track to the skin surface. The involucrum is often the first sign of osteomyelitis on radiographic examination (14).
SURGICAL VERSUS MEDICAL MANAGEMENT OF OSTEOMYELITIS
The requisite duration of antibiotic therapy following resection of osteomyelitis is unclear in the diabetic foot (16). This is, in large part, because of the lack of experimentally derived treatment duration. Currently, treatment relies heavily on physician preference, personal experience, and/or knowledge from case series (17).
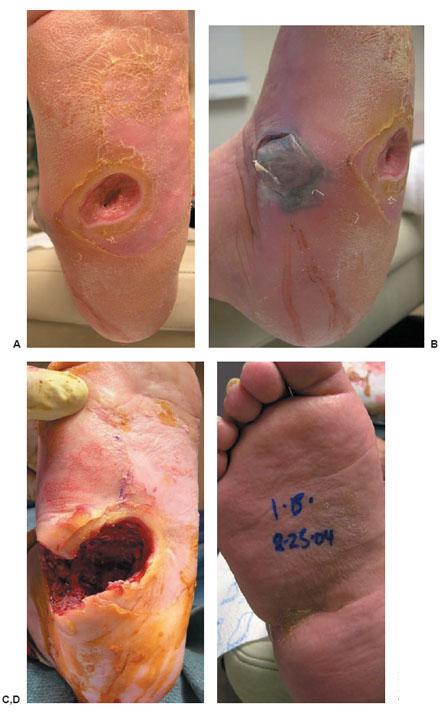
Figure 8.8 A–D. Exposed first metatarsal head (confirmed chronic osteomyelitis). Limited resection of metatarsal head removes infected bone and debulks the area to allow for flap closure. The acute soft tissue infection was resolved with initial débridement and 5 days of intravenous antibiotics while hospitalized.
Because of the wide range of recommendations within the literature by diabetic experts, antibiotic duration may range from 1 week to 6 months (18–20). Longer antibiotic durations are often employed because of the paucity of data showing infection recurrence rates for treatments shorter than 4 weeks (19,21). The Infectious Diseases Society of America (IDSA) has published guidelines that indicate four scenarios in which osteomyelitis in the diabetic foot can be treated without surgical treatment (22):
1. Surgical resection causes excessive loss of function
2. Unreconstructable vascular disease and trying to avoid amputation
3. Risk of surgery is too high because of medical conditions
4. Infection is confined to the forefoot
According to the IDSA, patients who present with any of these four scenarios are advised to follow a course of 3 to 6 months of parenteral antibiotics that may eventually be amenable to oral therapy (22).
However, if sepsis or purulence is present, immediate débridement of bone and soft tissues supersedes all of the preceding IDSA recommendations. Furthermore, as concern over antibiotic resistance worsens, caregivers should carefully consider the consequences of prolonged medical therapy, particularly in cases involving the forefoot. Among the causes of resistant organisms is extended antibiotic therapy (23). In fact, overuse of antibiotics has become a major public health threat, with antibiotic resistance spreading worldwide (23). MRSA, S. aureus with intermediate vancomycin resistance, and vancomycin-resistant enterococci are on the rise (24). At one time, a patient with osteomyelitis or a history of prior hospitalizations for the same wound was at the highest risk for MRSA (hospital-acquired resistance). However, with the rapid emergence of community-acquired strains, there is increased urgency to modify treatment paradigms to decrease the overall use of antibiotics. Microbiological data are available at all hospitals. It is helpful to have an understanding of resistance patterns given the significant rise of MRSA. Proper selection of empiric antibiotics should be based on recent local data, not historical dogma. A review of the Beth Israel Deaconess Medical Center, Boston, Massachusetts (BIDMC) antibiogram from July 1, 2006 to June 30, 2007 revealed only a 45% sensitivity rate to oxacillin and cefazolin for 1892 S. aureus isolates. Levofloxacin had a 42% sensitivity rate for S. aureus. Consequently, vancomycin had a 100% sensitivity rate (BIDMC Inpatient antimicrobial susceptibilities for aerobic bacteria, Caregroup performance manager, 7/1/2006 to 6/30/2007). However, a complete understanding of minimum inhibitory concentration (MIC) breakpoints, which is beyond the scope of this chapter, would alert the practitioner to consider alternative coverage for MRSA.
Traditionally, treatment for diabetic foot osteomyelitis has consisted of 6 weeks of parenteral antibiotics. However, treatment using long-term antibiotics has been disappointing given recurrence rates as high as 30% (22). Furthermore, the cost of long-term parenteral antibiotics can range from $3500 to $10,000 (20). Gordois et al in 2003 reported that the overall cost of treating a diabetic foot ulcer complicated with osteomyelitis is $45,579 (25).
Several studies have advocated the use of long-term antibiotics for osteomyelitis in the diabetic foot, an approach that is rarely employed at our institution. The often-cited article by Bamberger et al in 1987 studied 51 patients with osteomyelitis (26). All patients in the study received at least 4 weeks of intravenous antibiotics or had combined intravenous and oral antibiotics for 10 weeks. The authors stated that diabetic foot osteomyelitis in the absence of extensive necrosis or gangrene usually responds to antimicrobial therapy without the need for an ablative procedure. In this study, only two patients had bone biopsies performed. An additional six patients had specimens sent after a below-knee amputation and two were sent after partial amputation. Of the eight patients, all had pathologic evidence of osteomyelitis. Therefore, only 10 patients out of 51 had histopathologic evidence of osteomyelitis, which poses this question: Did the patients indeed have osteomyelitis? Tice et al in 2003 reviewed 454 patients who had osteomyelitis diagnosed by clinical and physical assessment, wound culture, and radiographs (27). In this study, antibiotics were administered for 25 to 28 days ± 10 to 15 days. Mean follow up was between 6 to 120 months. Three hundred fifteen patients (69%) were cured; 139 (31%) recurred. Of the recurrences, 56% recurred within 3 months, 78% recurred within 6 months, and 95% recurred within 13 months. All patients in the study did not have histopathologic-proven osteomyelitis.
The standard treatment for chronic osteomyelitis at BIDMC is surgical excision of the infected bone (Fig. 8.9A–C). The subsequent route and duration of antibiotic therapy depends on the type of bone infected. We have shown that when osteomyelitis is excised from the forefoot (metatarsal or toe), our cure rate is 94%. The mean duration of predominantly oral “mop-up” antibiotic therapy is 12.2 days (28). All specimens were confirmed histologically to be osteomyelitis. Not only will surgical resection of all necrotic bone decrease the duration of antibiotics, but also the bone culture is paramount to determine accurate speciation and resistance of the offending organism for proper antibiotic selection. The authors do not agree with other experts that forefoot osteomyelitis should be treated primarily with antibiotics alone (22,24). In the study by Embil, long-term use of oral antibiotics for osteomyelitis was advocated (24). Diagnosis of osteomyelitis was made by bone culture in only 27% of episodes, and never included pathologic examination. The remainder of cases were diagnosed through a variety of methods, including probe to bone and radiographic signs. Ninety-three percent of the sites were in the forefoot, a distribution similar to our previously reported data. However, they excluded patients if there was a prior abscess, acute osteomyelitis, or a resistant organism. Although it is encouraging that their data support the efficacy of oral antibiotics, for what has traditionally been regarded as a parenteral antibiotic disease, the mean duration of 40 ± 30 weeks of therapy is just too long.
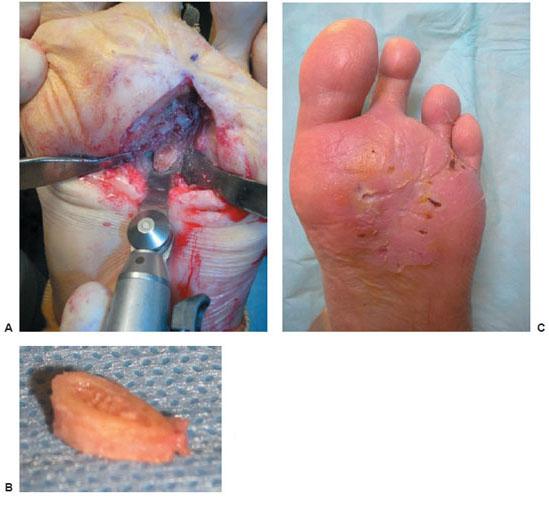
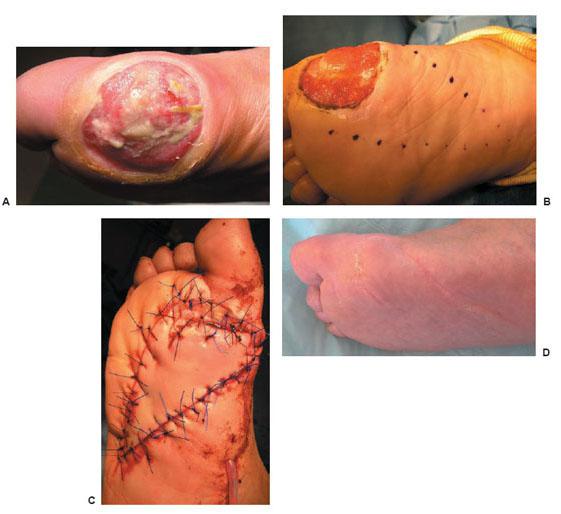
Figure 8.9 A–C. Complete surgical resection of osteomyelitis is essential for success. This is an example of resection of a clean bone margin following removal of the third metatarsal head. This patient received 12 days of organism-specific antibiotic and was primarily closed following excision of osteomyelitis.
Several studies support primary surgical resection of osteomyelitis (29–31). Henke et al found that of 237 patients with digital osteomyelitis, surgical débridement was associated with greater wound healing (odds ratio 3.9, 95% CI 1.2–4.2, p = 0.02). Patients who had prolonged preadmission antibiotics, and thus a delay in surgical management, were found to be associated with decreased wound healing (odds ratio 0.2, 95% CI 0.05–1.1, p = 0.07) and greater limb loss (odds ratio 0.34, 95% CI 0.15–0.77, p = 0.009) (29).
The importance of surgical resection is also seen in the study by Ha Van et al, which divided 67 patients into two groups (30). Group I had 35 patients with histopathologic evidence of osteomyelitis treated with antibiotic therapy, offloading, and wound care. Group II had 32 patients treated with the same medical treatment and conservative surgery consisting of limited resection of the infected part of the phalanx or metatarsal bone. The results showed that 20/35 (57%) patients healed in group I, and 25/32 (78%) patients healed in group II. The duration of healing in group I was 462 ± 98 days, and in group II was 181 ± 30 days. The duration of antibiotic therapy in group I was 246.9 ± 232 days and 111 ± 121 days in group II (12). This study showed that surgery would shorten healing times as well as decrease the need for long-term antibiotic therapy, thus limiting the emergence of resistant strains.
We are less certain about antibiotic duration in midfoot and rearfoot bones. Longer antibiotic durations and higher reinfection rates may be encountered. The anatomic variation and increased cancellous composition make it more difficult to determine with certainty that all infected bone has been resected. Simpson et al (31) further emphasize this point. The study prospectively followed 50 patients with osteomyelitis divided into three groups based on the “completeness” of surgical bone resection:
Group I: Wide excision of all necrotic bone with >5 mm of clean margins (n = 15, no recurrence)
Group II: Excision of all necrotic bone with 5 mm of clean margins (n = 29, 28% recurrence)
Group III: Intralesional biopsies with incision, drainage, and soft tissue débridement (n = 6, 100% recurrence)
All patients received 6 weeks of intravenous antibiotic therapy followed by 6 weeks of oral therapy. The mean length of follow-up was 26.2 months. Because the results showed that in group I there was no recurrence, and in group III all recurred within the first year, it emphasized the importance of complete excision of all necrotic bone with a higher degree of surgical clearance at the margin.
In conclusion, an aggressive surgical approach to osteomyelitis shortens healing times and decreases the need for long-term antibiotic therapy. This should help to limit the emergence of resistant bacteria, and greatly reduce both inpatient and outpatient costs. In the authors’ opinion, lower extremity osteomyelitis is primarily a surgical disease. Our 6% reinfection rate confirms that aggressive and expedient surgical resection of all infected tissue in conjunction with a brief course of an organism-specific antibiotic is successful. Specifically, when forefoot osteomyelitis has been completely resected, the authors have experienced excellent results with an average of 2 weeks of organism-specific, predominantly oral, antibiotics. Part of this success is because of expedient wound closure once infection has been eradicated by surgical débridement. Osteomyelitis involving bones with greater cancellous composition may require more extensive débridement and longer antibiotic durations.
REFERENCES
- Caputo GM, Cavanagh PR, Ulbrecht JS, et al. Assessment and management of foot disease in patients with diabetes. N Engl J Med 1994;331:854–860.
- Frykberg RG. An evidence-based approach to diabetic foot infections. Am J Surg 2003;186/5A:44S–54S.
- Tentolouris N, Jude EB, Smirnof I, et al. Methicillin-resistant Staphylococcus aureus: an increasing problem in a diabetic foot clinic. Diabet Med 1999;16: 767–771.
- Cook J, Cook E, Landsman AS, et al. A retrospective assessment of partial calcanectomies and factors influencing postoperative course. J Foot Ankle Surg 2007;46:248–255.
- Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med 1999;341:1906–1912.
- Tan JS, Friedman NM, Hazelton-Miller C, et al. Can aggressive treatment of diabetic foot infections reduce the need for above-ankle amputation? Clin Infect Dis 1996;23(2):286–291.
- Reiber GE. The epidemiology of diabetic foot problems. Diabet Med 1996; 13(Suppl 1):S6–S11.
- Lavery LA, Armstrong DG, Wunderlich RP, et al. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003;26:1435–1438.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;2:217–228.
- Reiber GE. Epidemiology of foot ulcers and amputations in the diabetic foot. In: Bowker JH, Pfeifer MA, eds. The Diabetic Foot, 6th ed. St. Louis: Mosby, 2001:13–32.
- Mackowiak PA, Jones SR, Smith JW. Diagnostic value of sinus tract cultures in chronic osteomyelitis. JAMA 1978;239:272–275.
- Lavery LA, Sariaya MS, Ashry H, et al. Microbiology of osteomyelitis in diabetic foot infections. J Foot Ankle Surg 1995;34:61–64.
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg 1999;177:282–286.
- Kumar V, Cotran RS, Robbins SL. The musculoskeletal system: osteomyelitis. In: Brown P, Herbranson E, eds. Basic Pathology. 6th ed. Philadelphia: Saunders, 1997:672.
- Blume PA, Dey HM, Daley LJ, et al. Diagnosis of pedal osteomyelitis with Tc-99m HMPAO labeled leukocytes. J Foot Ankle Surg 1997;36:120–126.
- Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Inf Dis 2004;39(2):S115–S122.
- Lipsky BA, Berendt AR. Principles and practice of antibiotic therapy of diabetic foot infections. Diabetes Metab Res Rev 2000;16:S42–S46.
- Rao N. Anti-infective therapy for foot ulcers in patients with diabetes. Clin Orthop Relat Res 2005;439:87–90.
- Armstrong DG, Lipsky BA. Diabetic foot infections: stepwise medical and surgical management. Int Wound J 2004;1(2):123–132.
- Shank CF, Feibel JB. Osteomyelitis in the diabetic foot: diagnosis and management. Foot Ankle Clin 2006;11(4):775–789.
- Pittet D, Wyssa B, Herter-Clavel C, et al. Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long-term follow-up. Arch Intern Med 1999;159:851–856.
- Lipsky BA, Berendt AR, Deery HG, et al, and the Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39(7):885–910.
- Hartemann-Heurtier A, Robert J, Jacqueminet S, et al. Diabetic foot ulcer and multidrug-resistant organisms: risk factors and impact. Diabet Med 2004;21: 710–715.
- Embil JM, Rose G, Trepman EM, et al. Oral antimicrobial therapy for diabetic foot osteomyelitis. Foot Ankle Int 2006;27(10):771–779.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003;26:1790–1795.
- Bamberger DM, Daus GP, Gerding DN. Osteomyelitis in the feet of diabetic patients. Long-term results, prognostic factors, and the role of antimicrobial and surgical therapy. Am J Med 1987;83:653–660.
- Tice AD, Hoaglund PA, Shoultz DA. Outcomes of osteomyelitis among patients treated with outpatient parental antimicrobial therapy. Am J Med 2003;114:723–728.
- Cook E, Basile P. Eliminating the need for prolonged antibiotics after surgical resection of forefoot osteomyelitis. Poster Presentation, presented at: American College of Foot and Ankle Surgeons Annual Meeting; 2008; Long Beach, CA.
- Henke PE, Blackburn SA, Wainess RW, et al. Osteomyelitis of the foot and toe in adults is a surgical disease: conservative management worsens lower extrimity salvage. Ann Surg 2005;241(6):885–894.
- Ha Van G, Siney H, Danan JP, et al. Treatment of osteomyelitis in the diabetic foot. Contribution of conservative surgery. Diabetes Care 1996;19(11): 1257–1260.
- Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br 2001;83(3):403–407.
RECOMMENDED READINGS
Consensus Development Conference on diabetic foot wound care: 7–8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care 1999;22:1354–1360.
Eneroth M, Apelqvist J, Stenstrom A. Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int 1997;18: 716–722.
Grayson ML, Gibbons GW, Balogh K. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995; 273(9):721–723.
Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis 1997;25: 1318–1326.
Peterson LR, Lissack LM, Canter K. Therapy of lower extremity infections with ciprofloxacin in patients with diabetes mellitus, peripheral vascular disease, or both. Am J Med 1989;86:801–808.
Swiontkowski MF, Hanel DP, Vedder NB. A comparison of short- and long-term intravenous antibiotic therapy in the postoperative management of adult osteomyelitis. J Bone Joint Surg Br 1999;81-B(6):1046–1050.
Vankatesan P, Lawn S, Macfarlane RM. Conservative management of osteomyelitis in the feet of diabetic patients. Diabet Med 1997;14:487–490.
Waldvogel FA, Medoff, G, Swartz, MN. Osteomyelitis: a review of clinical features, therapeutic considerations, and unusual aspects. N Engl J Med 1970; 282:198–206.
Yadlapalli NG, Vaishnar A, Sheehan P. Conservative management of diabetic foot ulcers complicated by osteomyelitis. Wounds 2002;14:31–35.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree