ATA low-risk papillary thyroid cancer (with all of the following):
No local or distant metastases
All macroscopic tumor has been resected
No tumor invasion of locoregional tissues or structures
The tumor does not have aggressive histology (e.g., tall cell, hobnail variant, columnar cell carcinoma)
If 131 I is given, there are no RAI-avid metastatic foci outside the thyroid bed on the first posttreatment whole-body RAI scan
No vascular invasion
Clinical N0 or ≤5 pathologic N1 micrometastases (<0.2 cm in largest dimension)
Intrathyroidal, encapsulated follicular variant of papillary thyroid cancer
Intrathyroidal, well-differentiated follicular thyroid cancer with capsular invasion and no or minimal (<4 foci) vascular invasion
Intrathyroidal, papillary microcarcinoma, unifocal or multifocal, including BRAFV600E mutated (if known)
ATA intermediate risk
Microscopic invasion of tumor into the perithyroidal soft tissues
RAI-avid metastatic foci in the neck on the first posttreatment whole-body RAI scan
Aggressive histology (e.g., tall cell, hobnail variant, columnar cell carcinoma)
Papillary thyroid cancer with vascular invasion
Clinical N1 or > 5 pathologic N1 with all involved lymph nodes < 3 cm in largest dimension
Multifocal papillary microcarcinoma with ETE and BRAFV600E mutated (if known)
ATA high risk
Macroscopic invasion of tumor into the perithyroidal soft tissues (gross ETE)
Incomplete tumor resection
Distant metastases
Postoperative serum thyroglobulin suggestive of distant metastases
Pathologic N1 with any metastatic lymph node ‡ 3 cm in largest dimension
Follicular thyroid cancer with extensive vascular invasion (>4 foci of vascular invasion)
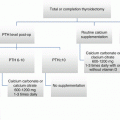
Following TL, completion thyroidectomy is not necessary for low-risk unilateral papillary thyroidectomy. Post TL, low-risk PTC meets all of the following criteria: no local or distant metastases, all macroscopic tumor has been resected, no tumor invasion of locoregional tissues or structures, the tumor does not have aggressive histology (e.g., tall cell, insular, columnar cell carcinoma), no lympho-vascular invasion, and clinical and pathologic N0 or ≤5 pathologic N1a micrometastases (<0.2 cm in largest dimension).
The primary arguments for TL include reduced perioperative morbidity and no difference in local recurrence or overall survival and less dependence upon thyroid hormone replacement. The primary arguments against TL include more dependence upon ultrasound surveillance, inability to use radioactive iodine (RAI), and potential need for completion thyroidectomy. The arguments for TT include no need for completion thyroidectomy, ease of surveillance with thyroglobulin (Tg), ability to administer RAI if needed, and treating any potential contralateral disease. The arguments against TT include increased perioperative morbidity (both recurrent nerves and all parathyroid glands at risk) and dependence upon thyroid hormone replacement.
Perioperative Morbidity
In recent studies, operative outcomes have been reported to be related to surgeon volume. Sosa et al. found that higher surgeon volume was associated with favorable patient outcomes, especially with regard to recurrent laryngeal nerve injury and wound complications [3]. This was clearly delineated for patients undergoing TT for thyroid cancer. In Sosa et al.’s cross-sectional analysis, all patients who underwent thyroidectomy in Maryland between 1991 and 1996 in a statewide hospital discharge database were included. Surgeons were stratified by thyroidectomy case volume per year, 1–9, 10–29, 30–99, and 100 or greater. High-volume surgeons performed the most total thyroidectomies of all the groups and were more likely to operate on patients with cancer. High-volume surgeons had significantly better outcomes than low-volume surgeons; they still had higher overall postoperative complication rates when performing TT compared to TL [3].
In a cross-sectional study of the Nationwide Inpatient Sample (the largest all-payer inpatient care database publicly available in the United States), 2003–2009, all adult patients who underwent TT and TL for benign or malignant conditions were included, and their surgeon volume was analyzed. Surgeon volume was categorized as low if they performed fewer than ten thyroid resections per year or high if performing >99 thyroid operations per year. 62,722 procedures were reviewed; most cases were TT (57.9 %) performed for benign disease. Undergoing TT had an increased risk of complications up to 20.4 % compared to TL, with a rate of 10.8 % (p < 0.0001). High-volume surgeons performed only 5.0 % of the surgeries overall, but 62.6 % of their procedures were TT. High-volume thyroid surgeons had a complication rate of 7.6 % following TL, and low-volume surgeons had a complication rate of 11.8 %. Regarding TT, high-volume surgeons had a complication rate of 14.5 %, and low-volume surgeons had a complication rate of 24.1 %. This was a significant finding (odds ratio 1.53, 95 % confidence interval 1.12, 2.11, p = 0.0083) that low-volume surgeons were more likely to have a complication after TT. Surgeons should counsel patients and should carefully consider the relative benefits and risks of TT vs. TL, even when high-volume surgeons perform surgery [4].
The surgical risks of TL followed by completion thyroidectomy are similar to those of a near-total or TT. Erdem et al. examined the outcomes of patients with differentiated thyroid carcinoma over a period of 8 years where 141 patients underwent completion thyroidectomy and 92 patients had primary surgery. The rate of permanent recurrent laryngeal nerve palsy and permanent hypoparathyroidism was similar between groups. They were 3.5 and 4.2 % in the completion thyroidectomy group, and 3.3 and 4.3 % in the primary TT group [5].
Surveillance: RAI Versus Long-Term Follow-Up with Ultrasound
Many centers are moving toward a more selective use of RAI coupled with a greater reliance on neck US and serial serum thyroglobulin (Tg) measurements for detection of recurrent disease. TT is necessary if the overall strategy is to include RAI therapy postoperatively, as would be the case for a known higher-risk tumor. However, more selective application of RAI and increased utilization of neck US have the potential to supplant the need for TT in low- and intermediate-risk patients that would have been performed in the past solely to facilitate RAI remnant ablation and follow-up. In addition, diagnostic whole-body RAI scanning is being used less and less, with a greater reliance on neck ultrasonography.
In a retrospective review by Vaisman et al. of 289 patients with mostly PTC >1 cm, patients were selected for either TL or TT without RAI remnant ablation and followed for recurrent or persistent structural disease with ultrasound. Changes in serum Tg were not helpful in identifying the presence of persistent/recurrent structural disease. Patients in this study with an identified recurrence were asymptomatic. The structural disease was identified on surveillance ultrasound, confirmed with biopsy, and did not have a corresponding increase in Tg [6]. In low-risk PTC treated surgically without RAI, ultrasound is the preferred modality for surveillance over Tg. Sonographic surveillance of the contralateral lobe is relatively straightforward; however sonographic surveillance of cervical nodes is more nuanced, and a lack of expertise in sonographic surveillance may limit utility of TL in some areas.
Overall Survival Differences Between TL and TT with Focused Selection Criteria
Applying selection factors to identify low-risk disease has demonstrated that in such patients the long-term outcomes are similar when treated with TL or TT. In the study by Bilimoria et al., data on extrathyroidal extension, completeness of resection, and other factors known to impact survival and recurrence were not available. It is unclear how often TL was done based on proper selection of low-risk PTC and how often TL was done in high-risk patients. Reasons for TL in a high-risk patient may include significant comorbid conditions, inability to obtain a complete resection, or concern over the status of the contralateral recurrent laryngeal nerve. In a study by Haigh, 7 % of TL patients had associated extrathyroidal extension, and 8 % had high-risk features as determined by the age, metastases, extent, and size (AMES) classification system. Further, 5 % of tumors were >5 cm and 1 % had distant metastasis [7]. In the TL patients analyzed by Mendelsohn, external beam radiation therapy was performed in 1–2 % [14]. In the published series by Haigh, Bilimoria, and Kiernan, 12–20 % of patients undergoing TL receive radioactive iodine therapy [7, 8, 9]. Thus, it is always difficult to generalize results from published series to individual patients. At the very least, patients interested in undergoing a less aggressive surgical approach such as TL must understand the potential need for intraoperative conversion to TT or completion thyroidectomy due to discovery of more advanced disease and potential need for radioiodine therapy.
New evidence shows that with proper patient selection, TL may be sufficient treatment in low-risk PTC patients. Adam et al. performed an updated analysis of 61,775 patients in the National Cancer Database who underwent thyroid surgery between 1998 and 2006. They demonstrated that the overall survival advantage seen for patients with 1–4 cm PTC who underwent TT in the Bilimoria study disappeared when further adjustment was made for additional variables related to complexity and severity of illness. Patient comorbidities, tumor multifocality, extrathyroidal extension, nodal disease, distant metastases, and completeness of resection were taken into account. Similarly, no overall survival advantage was seen when the group was subdivided into patients with 1–2 cm and 2–4 cm PTC [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree