Clinical
Rapid nodule growth
Family or personal history of predisposition syndrome (e.g., PTEN hamartoma tumor, APC-associated polyposis, RET associated)
Childhood exposure to ionizing radiation
Younger (<18 years) or older (>50 years) age
Men
Larger nodule size
Imaging
Ultrasound features (especially taller-than-wide shape, microcalcifications, increased intranodular vascularity)
FDG-PET avidity
Molecular
Galectin-3- or HBME-1-positive on immunocytological analysis
TSHR mRNA detectable in peripheral blood
Gene expression classifier or micro-RNA panel testing of biopsy is “suspicious”
Detection of somatic mutation or rearrangement (e.g., BRAF V600E, RAS) in preoperative biopsy using conventional sequencing or next-generation sequencing
Surgical options include diagnostic thyroid lobectomy with isthmusectomy or total thyroidectomy (Table 10.2). Nodulectomy is not indicated. Voice changes are a risk of thyroid surgery, have been reported in 30–80 % of post-thyroidectomy patients, and are usually transient [21, 22]. There is a 1–5 % risk of recurrent laryngeal nerve palsy and 1–2 % risk of recurrent laryngeal nerve paralysis following thyroidectomy [7, 23]. Total thyroidectomy is associated with the rare (likelihood of ~1 in 1000) risk of bilateral recurrent laryngeal nerve injury necessitating tracheostomy. After total thyroidectomy, the risk of temporary hypocalcemia is 1–14 % and can be permanent in 1–6 % of patients [23]. Postoperative cervical hematoma requiring reoperation can be life-threatening and cause airway compromise but is infrequent occurring in ~1 % of post-thyroidectomy patients [24–26]. The type and incidence of risks following completion thyroidectomy are similar to that of a near-total or total thyroidectomy [27, 28]. L-Thyroxine supplementation is required in all patients following total thyroidectomy and in 15–30 % of patients following thyroid lobectomy [29, 30]. Surgeon experience influences the risks of thyroidectomy, and in population-level analysis, higher-volume surgeons have lower overall complication rates [31, 32].
Table 10.2
Initial surgical options for indeterminate nodule
Pros | Cons | Indication | |
|---|---|---|---|
Thyroid lobectomy | Limits risks of hypoparathyroidism and bilateral recurrent laryngeal nerve injury | Second operation for completion thyroidectomy necessary if histology indicates | Preoperative concern for low-risk thyroid cancer |
70–85 % avoid permanent hypothyroidism | Patient preference | ||
Total thyroidectomy | Provides initial oncologic surgery in preparation for radioactive iodine ablation when indicated | Permanent hypothyroidism | Preoperative concern for high-risk thyroid cancer and need for post-op radioactive iodine ablation/treatment |
Increased operative risks including risk of permanent hypoparathyroidism and bilateral recurrent laryngeal nerve injury | Concurrent clinical features such as contralateral dominant nodule, existing hypothyroidism, family, or personal history of predisposition syndrome | ||
Patient preference |
The decision regarding extent of initial surgery is influenced by a number of clinical factors including preexisting thyroid dysfunction, presence of a contralateral dominant thyroid nodule, and patient preference [7]. The presurgical likelihood of malignancy is also an essential component in deciding the extent of initial surgery. Due to the decreasing use of radioactive iodine ablation, the 2015 American Thyroid Association guidelines now recommend thyroid lobectomy for thyroid cancers <1 cm without extrathyroidal extension or lymph node metastasis. Total thyroidectomy or lobectomy can be considered for thyroid cancers 1–4 cm that do not have extrathyroidal extension or lymph node metastasis, while total thyroidectomy is definitively indicated for cancer >4 cm or for cancer of any size with gross extrathyroidal extension, clinically apparent nodal, or distant metastases. Therefore, counseling patients on the appropriate extent of initial surgery requires accurate preoperative assessment of malignancy risk in addition to consideration for the likelihood that the malignancy may be associated with more aggressive biologic behavior.
After lobectomy, completion thyroidectomy is recommended for patients with histologic malignancy who would have required total thyroidectomy if the diagnosis was known preoperatively. Completion thyroidectomy and radioactive iodine ablation should be performed for malignancies with a higher risk of structural recurrence such as patients with American Joint Committee on Cancer advanced stage III/IV disease or patients with incomplete tumor resection or bulky clinical lymphadenopathy [7, 33]. Preoperative vocal fold assessment with either direct laryngoscopy or transcutaneous laryngeal ultrasound should be performed prior to any reoperative surgery [7, 34, 35].
Clinical Risk Factors
Clinical variables such as age, gender, and the presence of unique symptoms or examination findings have been examined with regard to their ability to predict malignancy. Traditionally, an elevated concern for cancer has been associated with a history of rapid nodule growth; family history of thyroid cancer or inherited predisposition syndrome such as RET associated, PTEN hamartoma tumor, or APC-associated polyposis syndromes; or childhood exposure to ionizing radiation. On physical exam, fixation of nodule to surrounding neck structures and palpable lymphadenopathy are also concerning features.
The ability of clinical characteristics of patients with indeterminate FN/SFN cytology to predict malignancy was examined by Baloch et al., and factors associated with malignancy included age >40 years, nodule size >3 cm, and male gender [36]. The association between gender and an increased risk of malignancy has been inconsistent, but in a meta-analysis inclusive of 19 studies with “indeterminate” nodules, men had a 1.5-fold higher risk for malignancy compared to women [37]. In the subset of nodules with Hürthle cell neoplasm cytology, men have been shown more consistently to have a higher risk of cancer [38–40]. Interestingly, in one of the larger published series of 603 follicular and Hürthle cell neoplasms from Italy, gender was not associated with cancer risk but women compared to men had a higher incidence of histologic malignancy with extrathyroidal extension [38].
The association between age and malignancy has also been variable, and increased risk has been seen with both younger and older patients [41]. In another large single institution series of 639 patients with indeterminate nodules, Banks et al. observed that age was an independent predictor of malignancy on multivariate analysis; patients’ age <50 years had a 3 % increase in risk of cancer for each year younger in age, and patients’ age >50 years had a 3.4 % increase in cancer risk for each year older in age [42]. An age of 50 years has also been shown to be the threshold for increased cancer risk in other studies [39, 43]. Pediatric patients (age ≤18 years) have an overall higher risk of malignancy associated with thyroid nodules (15–30 %), and the risk can be up to 50 % in nodules with indeterminate cytology [44, 45]. The 2015 American Thyroid Association guidelines for pediatric patients recommend surgery for all indeterminate FNA results in children [46].
Larger nodule size has been reported to be associated with a higher risk of malignancy especially for follicular neoplasms and Hürthle cell neoplasms [47]. In a study of 149 nodules with FN/SFN cytology, Tuttle et al. found that the risk of malignancy was ~threefold higher in nodules >4 cm as measured by palpation [48], while Baloch et al. found that ≥3 cm was associated with a twofold greater risk of malignancy in a single institution series of 184 follicular neoplasm nodules [36]. In a consecutive series of 55 patients with Hürthle cell neoplasms, malignancy increased with nodule size and older patient age [49]. Nodule size was another predictor of malignancy in Banks et al., and indeterminate nodules that were 2.5 cm had the lowest risk of cancer. A higher risk of cancer was associated with smaller indeterminate nodules (53 % increase in risk for every 1 cm decrease in size) and with larger nodules (39 % increase in risk for every 1 cm increase in size) [42]. Surgery for relief of compressive symptomatology may also be indicated for nodules >3 cm as these have been associated with globus symptoms on multivariate analysis [50].
Imaging Risk Factors
Thyroid ultrasound has been widely used for nodule risk stratification and is used to identify non-palpable nodules, define nodule features, and characterize cervical lymph nodes. In studies inclusive of all thyroid nodules, ultrasound features that increase concern for cancer includes marked hypoechogenicity, spiculated or ill-defined margin, taller-than-wide shape, and microcalcifications [51]. However, whether these features are useful in nodules with indeterminate cytology remains unclear. In a study of 180 patients who had thyroid nodules with indeterminate cytology (follicular neoplasm, Hürthle cell neoplasm, and SUSP), a taller-than-wide shape was associated with 99 % specificity and 92 % positive predictive value for malignancy. When ≥2 concerning ultrasound features were present, the risk of malignancy was >70 % [52]. Taller-than-wide shape was also shown to be associated with cancer in a series of 61 patients who all had thyroidectomy for AUS/FLUS cytology [53]. But in another study of 505 follicular and Hürthle cell neoplasms, malignancy was associated with only microcalcifications [54].
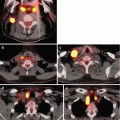
In a meta-analysis by Brito et al., nodules with indeterminate cytology were evaluated in a subset analysis; they found that suspicious features did not accurately predict malignancy with the exception of increased intranodular vascularity, which was likely due to the increased frequency of follicular neoplasms in the study population [55]. Use of color Doppler to evaluate intranodular vascularity may be helpful for evaluating follicular neoplasms as it is theorized that these contain higher cellularity and variation in echogenicity and internal vascularity compared to PTC [56] (Fig. 10.1). However, interpretation of flow on color Doppler may be associated with up to 30 % interobserver variability, limiting its utility.
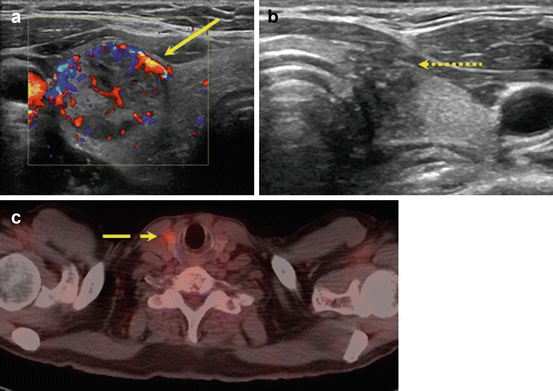

Fig. 10.1
Imaging features that increase concern for malignancy in indeterminate nodules. Ultrasound features including intranodular hypervascularity (a, solid line) and taller-than-wide shape (b, dotted line) have been associated with an increased risk of malignancy in nodules with indeterminate cytology. Avidity on fluorodeoxyglucose-positron emission tomography (FDG-PET) (c, dashed line) may also increase cancer risk, but the high cost of FDG-PET scans limits their widespread use as part of diagnostic evaluation
Improving preoperative risk stratification has also been studied using other imaging modalities such as fluorodeoxyglucose-positron emission tomography (FDG-PET) (Fig. 10.1). In a systematic review inclusive of six articles that examined accuracy of FDG-PET to diagnose thyroid cancer, the pooled specificity and sensitivity were 95 % and 48 %, respectively [57]. Thus, an FDG-avid indeterminate nodule is not always malignant, but malignant nodules are rarely non-FDG avid. Broad applicability of FDG-PET scans is complicated by inconsistent definitions for FDG avidity, low imaging resolution for small nodules, and selection bias. Furthermore, FDG-PET scanning is costly overall, although a cost-effectiveness study using reimbursement rates from the Netherlands demonstrated that routine use of PET could reduce unnecessary surgeries and was associated with lower costs than other diagnostic adjuncts such as molecular testing [58].
Real-time elastography measures the tissue displacement when external force is applied to the thyroid nodule of interest. The imaging relies on the assumption that thyroid cancers are firmer than benign nodules, and as a result, calcifications and cystic nodules can cause diagnostic inaccuracies. Although small single institution studies have demonstrated high sensitivity (up to 96 %) and specificity (up to 95 %) in detecting cancer, high interobserver variability in addition to non-standardized methodologies has limited its use. In a multi-institutional study inclusive of 498 nodules, the addition of elastography to ultrasound appeared to increase sensitivity for malignancy which could potentially better identify nodules that do not require FNA biopsy. For nodules with indeterminate cytology, elastography results appear to be poorly predictive of histology [59, 60].
Molecular Risk Factors
A number of adjunctive molecular tests have been evaluated to determine their utility in diagnosing malignancy in indeterminate nodules. Immunocytochemical analysis of markers expressed predominantly in PTC such as CK-19, galectin-3, and HBME-1 have been studied [61]. Galectin-3 staining in preoperative cytology was specifically evaluated in a multi-institutional study of 465 follicular neoplasms and accuracy was 88 % [62]. Serum markers such as thyroid-stimulating hormone receptor (TSHR) mRNA or thyroglobulin (Tg) can be measured in peripheral blood and are another area of investigation. When TSHR mRNA is detected in patients with follicular neoplasm nodules, accuracy for predicting malignancy was 85 %. In an algorithm that incorporated TSHR mRNA positivity, nodule size (<3.5 cm or ≥3.5 cm), and number of suspicious ultrasound characteristics (hypervascularity, microcalcifications, irregular shape, and indistinct margins), the diagnostic discrimination for indeterminate FNA results was increased to 91 % accuracy, 97 % sensitivity, 95 % negative predictive value, 84 % specificity, and 88 % positive predictive value. In a series of 164 indeterminate nodules, elevated preoperative basal serum Tg levels were an independent predictor of malignancy [63]. These results have not been consistent, and in another smaller study of 39 patients with follicular or Hürthle cell neoplasms, Tg levels were poorly predictive [63]. Preoperative Tg levels are difficult to interpret especially in the presence of detectable Tg antibodies and should not be routinely used to assess malignancy risk.
The Afirma diagnostic test is a gene expression classifier (GEC) that measures the expression of 167 gene transcripts. The expression pattern was selected to be predictive of benign nodules and in a prospective, multicenter trial demonstrated a relatively high sensitivity (92 %) that translated into a negative predictive value of 93 % [64]. However, in nodules with SUSP cytology, the high malignancy rate resulted in an unreliable negative predictive value of 85 %, and thus GEC was not recommended for cytologically SUSP nodules. The GEC panel has a low specificity of 52 %, and diagnostic thyroidectomy is still needed when cytology results are “suspicious” even though many such nodules will be histologically benign. In Marti et al., GEC test performance was evaluated at two separate tertiary care centers with different cancer prevalences [65]. With the goal of obtaining a calculated negative predictive value >94 % and using the sensitivity and specificity data from the Alexander et al. multi-institutional study [64], Marti et al. determined that the pretest cancer risk of an indeterminate nodule should be 15–21 % for GEC to be clinically useful [65]. In this range, the cancer risk when GEC is “suspicious” is only 25–32 %. These findings further underscored the evolving observation that published negative and positive predictive values are not uniformly applicable across institutions or practices and vary according to cancer prevalence and risk of malignancy for each BSRTC category [66].
The second molecular testing modality frequently evaluated is the identification of somatic mutations and rearrangements. Activation of MAPK and PI3K-AKT pathways via cell-membrane receptor tyrosine kinases (RET, NTRK1) and intracellular signal transducers (BRAF, RAS) are known initiators of thyroid carcinogenesis [67, 68]. In PTC, BRAF V600E is the most common somatic mutation with an incidence that varies geographically in the USA from 40 to 50 % but up to 80 % of PTCs in Asia. Identification of BRAF V600E in indeterminate nodules can improve preoperative detection for PTC, and in a meta-analysis inclusive of 47 studies reporting use of BRAF V600E analysis in FNA biopsy specimens, a pooled specificity could not be calculated for the meta-analysis, but was 100 % when reported by selected studies. Pooled sensitivity in the indeterminate category inclusive of AUS/FLUS, FN/SFN, and SUSP nodules was 30 % (range 11–50 %) [69]. Therefore, BRAF V600E testing alone was not sufficient to reduce the need for diagnostic surgery altogether.
An alternative to single gene testing is to use a multigene panel such as the 7-gene panel inclusive of RAS (H-, N-, K–RAS codons 12, 13, and 61), BRAF (V600E and K601E), RET/PTC, and PAX8/PPAR rearrangements. In a validation study by Nikiforov et al., 513/1056 nodules with indeterminate cytology and histology had correlative molecular results using such panel. All BRAF-, RET/PTC-, and PAX8-/PPARG-positive cytology specimens were histologically thyroid cancers (specificity 100 %); however, RAS positivity was associated with an 85 % risk of malignancy [70]. A subsequent prospective study evaluating the clinical and real-time utility of the 7-gene panel demonstrated that the added specificity associated with using mutation testing was helpful for guiding extent of initial surgery such as total thyroidectomy for thyroid cancers that on histology were >1 cm in size. Therefore, the need for two-stage thyroidectomy was reduced in 30 % of patients [71]. However, the low sensitivity associated with the 7-gene panel resulted in a rate of malignancy associated with “negative” results that were too high to allow for surveillance only.
Newer multigene panels utilizing next-generation sequencing have incorporated additional genetic alterations that have since been identified in thyroid cancer. For example, one such panel (ThyroSeq) evaluates 14 genes, 42 gene rearrangements, and additional expression analysis of 8 genes used to assess for quantity and type of cells. Nikiforov et al. described the use of this broad panel in 143 follicular neoplasm nodules; 91 were studied retrospectively, and 52 were studied prospectively. Malignancy was diagnosed in 27 %, including 26 FV-PTC and 6 FTC. The overall sensitivity and specificity were 90 % and 92 %, respectively [72]. These performance parameters were replicated in a series of 98 AUS/FLUS nodules from the same institution [73]. Another test combines the 7-gene panel with a panel of 10 micro-RNAs (ThyGenX and ThyraMir), and in a study of 109 nodules with AUS/FLUS or FN/SFN cytology (32 % cancer prevalence), the combined panel sensitivity and specificity were 89 % and 85 %, respectively [74]. Independent validation studies for all of these newer molecular tests are still needed.
The detection of a genetic mutation or rearrangement may be helpful in predicting the presence of thyroid cancer, but the type of genetic alteration may also provide information on the thyroid cancer type and biologic behavior. The presence of BRAF V600E has been shown in some studies to be associated with an increased risk of PTC recurrence due to its association with lymph node metastasis and advanced stage disease [75]. However, these results have not been consistent in all studies [76, 77]. In a study inclusive of 1,510 patients who had 7-gene panel testing and treatment for thyroid cancer, RET/PTC rearrangement had the highest association with distant metastases. RAS-positive tumors were usually encapsulated FV-PTC, however; some aggressive tumor types were also seen with RAS mutations including medullary thyroid cancer and poorly differentiated/anaplastic cancers [78]. The multigene panel ThyroSeq has additional markers that may be associated with more aggressive variants of thyroid cancer including TERT and p53, and refinement of thyroid cancer phenotype may be obtained with this test although this is still being evaluated [79, 80].
The use of molecular testing for indeterminate nodules should consider the test strengths and planned clinical management. For example, the GEC can help identify nodules that may be more likely to be benign. Therefore, GEC does not provide any added utility if active surveillance is not a clinical consideration due to patient preference, associated symptoms, or presence of other concerning clinical or radiographic features. The 7-gene panel may help identify nodules with a higher risk of cancer and guide extent of thyroidectomy. Thus, such panel will not add to clinical decision-making if total thyroidectomy is already indicated due to preexisting thyroid dysfunction, contralateral dominant nodule, or patient preference. No study to date has yet determined the clinical or cost efficacy when using >1 molecular test on the same nodule.
Clinical Management Recommendations
AUS/FLUS
The incidence of an AUS/FLUS diagnosis ranges from 5 to 20 %, and the risk of cancer in an AUS/FLUS nodule is 15.9 % but ranges in studies from 5 to 40 % [8]. AUS/FLUS nodules with cellular atypia compared to a predominant microfollicular pattern have been observed to have a higher risk of malignancy [81]. Because of the highly variable incidence and malignancy rates associated with this cytology category, consideration of institutional and geographical rates should be incorporated into patient management recommendations. Repeat FNA can be benign in 40–50 % of nodules and should be considered in nodules that lack concerning clinical or imaging features [18, 19]. A repeated AUS/FLUS biopsy carries a 30–50 % risk of cancer, and either thyroid lobectomy or total thyroidectomy should be considered.
Molecular testing can certainly be considered if clinically indicated, and its accuracy is dependent on the prevalence of cancer in the tested population of nodules [66]. When AUS/FLUS biopsy results are GEC benign or ThyroSeq negative, the risk of cancer has been reported to be 3–4 %. According to recent National Comprehensive Cancer Network guidelines, if the cancer risk of an indeterminate nodule can be lowered to be equivalent to that of a cytologically benign nodule, then active surveillance can be considered [82]. GEC-suspicious or gene-positive nodules should undergo either thyroid lobectomy or total thyroidectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree