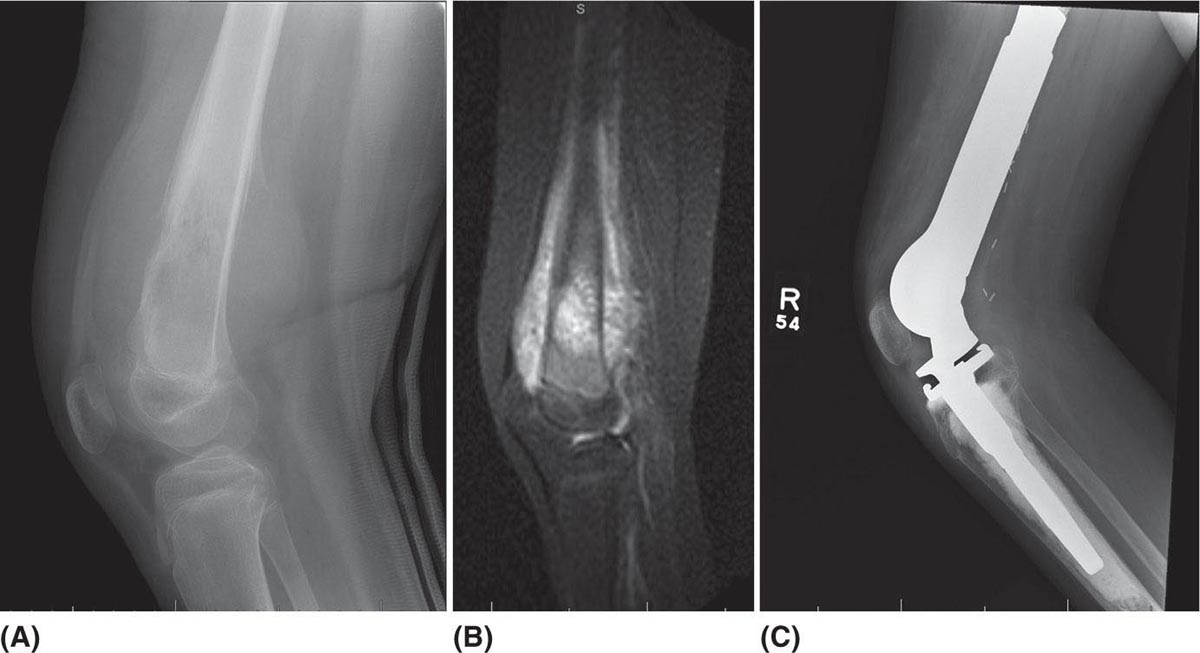
535 Surgical Approach and Surgical Reconstruction Options in Treatment of Bone Sarcomas Patients with sarcoma of the musculoskeletal system require complex care through an extensive multidisciplinary team. One critical component of this care team is an orthopedic surgeon with specialization in musculoskeletal oncology. Orthopedic oncologists provide surgical oncologic expertise and have proficiency in the various limb reconstruction techniques utilized to optimize patient outcome and function. Whether the surgical approach is limb salvage or amputation, sarcoma patients have unique lifelong musculoskeletal needs. These patients often require extensive physical therapy, prosthetics, orthotics, and possibly revision surgery. This chapter describes the critical components of the initial evaluation, biopsy, and surgical treatment options used to treat patients with bone sarcomas. It reviews some of the critical tumor characteristics, patient-specific factors, and procedure-specific rehabilitative requirements that can impact these complex surgical decisions. limb salvage, limb reconstruction, amputation, surgical approach, biopsy, bone sarcoma biopsy, bone sarcomas, healthcare providers, limb reconstruction techniques, musculoskeletal oncology, optimize patient outcome, physical therapy, prosthetics, surgical treatment Biopsy, Health Personnel, Musculoskeletal System, Patient Outcome Assessment, Physical Therapy Modalities, Prostheses and Implants, Reconstructive Surgical Procedures, Sarcoma INTRODUCTION Patients with sarcoma of the musculoskeletal system require complex care through an extensive multidisciplinary team. One critical component of this care team is an orthopedic surgeon with specialization in musculoskeletal oncology. Orthopedic oncologists provide surgical oncologic expertise and have proficiency in the various limb reconstruction techniques utilized to optimize patient outcome and function. Whether the surgical approach is limb salvage or amputation, sarcoma patients have unique lifelong musculoskeletal needs. These patients often require extensive physical therapy, prosthetics, orthotics, and possibly revision surgery. Therefore, a basic familiarity with these complex surgical procedures and their potential complications will assist all practitioners in optimizing patient care in sarcoma treatment. The primary goal of this chapter is to familiarize healthcare providers with the critical components of the initial evaluation, biopsy, and surgical treatment options used to treat patients with bone sarcomas. We also review some of the critical tumor characteristics, patient-specific factors, and the procedure-specific rehabilitative requirements that can impact these complex surgical decisions. INITIAL EVALUATION The initial evaluation of a patient with a suspected bone sarcoma begins with a relevant history and physical exam. Though simple and easily performed, the importance of this step can often be overlooked. Imaging assessment of a bone sarcoma typically begins with a radiograph followed by a variety of advanced imaging modalities. Each imaging modality can provide different information regarding the tumor. Following adequate imaging, a well-planned and executed biopsy is the next step in the diagnosis. The goal of this biopsy is establishing a definitive diagnosis, subtype, and grade, while providing adequate specimen for any additional pathologic testing. While the staging process begins with the physical exam, this should be completed with appropriate body imaging when a diagnosis of sarcoma is confirmed. History The history of present illness and past medical history of the patient is critical in the initial evaluation. This begins with patient-derived history regarding duration of symptoms, intensity and characteristics of pain, growth rate, and functional limitations. A history of any local trauma or injury should be elicited, as well as associated systemic symptoms, such as fevers and weight loss. The history should also include all comorbid conditions, relevant previous procedures (including biopsy), and medications. A personal and/or familial history of cancer may be contributive and should be noted. A thorough understanding of the patient’s baseline functional status is critical and should include any preexisting limitations or perceived dysfunction. At this first encounter, the surgeon should inquire about the patient’s current activity level and occupation. As musculoskeletal sarcoma frequently occurs in adolescents and young adults, it is also critical to explore their physical and occupational goals, as this may be impacted by the planned surgery and/or reconstructive option. Physical Examination While the focus of physical examination will be the mass or painful extremity, some general exam findings are critical. Body habitus, gait patterns, need for assistive device, and patient affect are immediately 54apparent examination findings and can impact surgical decision-making. Pathologic fractures can occur in association with bone sarcomas. Pathologic fractures at diagnosis or during neoadjuvant treatment have a prevalence of 5% to 10% in relation to osteosarcoma.1 Therefore, any deformity and/or significant change in pain symptoms should be evaluated with radiographs both on initial evaluation and throughout neoadjuvant treatments. If present, palpable masses should be well characterized with regard to firmness, mobility, compressibility, and temperature. Previous scars and biopsy sites should be carefully examined, with the orientation, length, and location well documented. Any skin changes (erythema, fungation, telangiectasias, seromas, or hematomas) associated with the tumor or biopsy site should be noted. A comprehensive vascular, lymphatic, and neurologic examination of the affected extremity may reveal edema, lymphadenopathy, vascular findings, or neurologic findings associated with tumor involvement or mass effect. Imaging Comprehensive imaging of the involved extremity plus appropriate staging studies should be performed during initial evaluation of all bone sarcomas. As imaging is covered in detail in Chapter 7, Radiologic Evaluation of Bone and Soft Tissue Sarcoma at Diagnosis and Follow-Up, the role of imaging is addressed subsequently as it relates to surgical planning. Imaging begins with orthogonal radiographs of the entire bone involved with the joint above and below. Orthogonal radiographs that are well taken can allow localization of bone tumors, while also providing information on aggressiveness and matrix formation. Aggressive bone lesions are characterized by a wide zone of transition with possible cortical destruction and intense periosteal reaction.2 This contrasts with less aggressive lesions, which have narrow zone of transition with little to no effect on the cortex and nonaggressive or absent periosteal reaction.2 Complete radiographic imaging of the bone allows for evaluation of preexisting deformity of the bone, osteoarthritis, osteopenia, or other musculoskeletal pathologic changes that may affect surgical planning. Although one can gain significant information from radiographs, additional cross-sectional imaging is still needed. MRI with and without contrast is used for further detailed localization of the tumor, evaluation of any possible soft tissue components, tumor heterogeneity, and relative location to vital structures including neurovascular structures.3 It is felt that unenhanced T1-weighted sequences are most suitable for evaluation of the tumor margins within the bone without clinically significant under- or overestimation.4 Obtaining an MRI scan of the entire bone involved is also important for evaluation of skip lesions or a discontinuous lesion within the same bone.3 MRI allows for detailed evaluation of any vessel encasement or infiltration that may preclude limb-salvage surgery. CT scan is an additional cross-sectional imaging technique that can aid the surgeon in preoperative evaluation, with additional bone detail needed for surgical planning. BIOPSY After all initial local imaging studies have been completed, a biopsy is performed for tissue diagnosis. A biopsy is viewed as the final diagnostic procedure, not a shortcut to a diagnosis.5 In this section are discussed specific principles that should be followed when performing a biopsy for musculoskeletal sarcoma. When a biopsy is improperly performed, it can have significant negative effects to the patient and can alter his or her surgical options.6–8 Biopsy-related errors are minimized through discussion of a multidisciplinary team, especially involving the orthopedic oncologist.6,9,10 Various effective biopsy techniques for the management of sarcoma have been described, including office-based biopsy, image-guided biopsy, and open biopsy in the surgical suite. Image-guided biopsy is typically performed by musculoskeletal radiologists, and prior to this biopsy, there should be discussion between the radiologist and the orthopedic oncologist regarding the location of biopsy. Communication with the pathology department is also important, as the pathologist has critical insight regarding the biopsy technique, tumor volume, and special tissue processing needs. As more targeted medical strategies are employed, the role of the treating medical oncologist in biopsy planning becomes more apparent. Nonroutine tumor testing, additional tumor volume, or specific tissue processing may be requested by the medical oncologist, particularly for resistant tumors or patients eligible for novel therapies or clinical trials. Biopsy Principles Biopsy should be performed after all imaging studies have been completed, so two important questions can be addressed prior to biopsy: (a) What portion of the sarcoma should be biopsied? and (b) What is the safest anatomic route to the location?11 55The biology of sarcomas, which are mesenchymal based, is much different from that of carcinomas. Sarcomas usually possess regional morphologic variations.12–14 Sarcomas grow in a centripetal fashion, with the most immature portion in the peripheral portion and often a central necrotic portion. This immature portion is also surrounded by a reactive zone of tissue.11 After meticulous imaging review by a radiologist and an orthopedic oncologist, who are familiar with the characteristics of sarcoma, usually viable and diagnostic portion of the tumor can be identified for biopsy. Given the heterogeneous nature of sarcoma, multiple biopsy samples are taken to help reduce the chances of “sampling error.” The extraosseous portion of the bony sarcoma is representative of the primary pathology and should be biopsied, if it exists.8 If no extraosseous portion exists, a cortical window can be created to obtain adequate tissue; if this is done poorly, it can place the patient at increased risk for pathologic fracture due to weakening of the structural integrity of the bone.15 After a specific portion of the tumor has been identified for biopsy, the approach to that portion must be identified. Traditional teaching has stressed the following two main principles: (a) The biopsy tract should be in line with the planned incision of definitive surgery. This allows for excision of the biopsy tract during definitive surgery. The thought is that the biopsy tract is seeded with tumor and should be excised to decrease the risk of local recurrence.8 (b) The biopsy tract should be the shortest route to the tumor, violate only one compartment, and be as distant as possible from vital neurovascular structures.16 Evidence has proposed that biopsy of musculoskeletal sarcoma should be performed at the institution of planned definitive treatment. This can decrease morbidity to the patient, can avoid treatment interruption, and can limit delays in diagnosis.6,17 Mankin et al. reviewed the complications with biopsies for sarcoma performed at referring centers as compared to treating centers.6 Biopsies for sarcoma performed at referral centers led to 27.4% errors in diagnosis, 13.9% were nonrepresentative, 36.3% led to change in treatment plan, and 17.4% changed the course or outcome of treatment. This is in stark contrast to biopsies performed at the treatment center, where 12.3% led to an error in diagnosis, 3.5% were nonrepresentative, 4.1% led to a change in treatment plan, and 3.5% changed the course or outcome of treatment. Biopsy Technique After anatomic location of the biopsy is determined, quality tissue must be obtained to allow for tissue diagnosis. There are various ways to obtain tissue with differences in accuracy. The diagnostic accuracy of fine-needle aspiration (FNA), core-needle biopsy, and open biopsy has been investigated. After review of the literature, Traina et al. found that core-needle biopsy was more accurate than FNA, and open or incisional biopsy showed the highest accuracy of all.8 Core-needle biopsies allow for better preservation of tumor architecture, leading to increased information from the biopsy tissue, as compared to FNA.11 Percutaneous core-needle biopsies have the advantage of decreased cost and lower risk of contamination, while providing diagnostic accuracy between 74% and 100%.8,18 Percutaneous core-needle biopsies appear to be most suitable for diagnosis of bone tumors. The use of image-guided biopsy can also increase diagnostic accuracy in deep musculoskeletal tumors.19 In the setting of a palpable sarcomatous mass, office-based core-needle biopsies have shown an accuracy of 91% and were found to be safe and well tolerated.20 If diagnosis cannot be obtained from percutaneous biopsy, an open incisional biopsy is indicated.21 Recent literature has also questioned the traditional teaching that the biopsy track must be excised and must not cross multiple compartments when performing coaxial core needle biopsies.22 Binitie et al. reviewed 59 soft tissue sarcoma patients who had undergone core-needle biopsies without excision of biopsy track at definitive treatment and saw no increase in local recurrence or rates of metastasis.23 As compared to percutaneous biopsy tracks, open incisional biopsy track scars are more clear and usually excised on definitive surgical treatment.22 SURGICAL TREATMENT The primary goal of surgical treatment is complete sarcoma resection with negative margins. The risk of local recurrence in osteosarcoma is significantly increased in patients who undergo resection with inadequate margins.24–26 While negative margin status is the primary surgical oncologic goal, there remains debate on the optimal size of this margin. In the current literature, this margin can vary between specific bone sarcomas, tumor grade, and tumor location.27,28 The secondary goal of surgery is to preserve and/or restore optimal function for the patient. The surgeons should be mindful to ensure it is the patients’ definition of optimal function that guides this critical discussion. During surgical 56planning, the patient and his or her support system should be educated about the realistic expectations of the postoperative course and resulting function of the limb. Furthermore, the patient’s ability or willingness to comply with postoperative precautions or rehabilitative requirements may influence the planned surgery or the favored reconstructive option. Complete resection with negative margins can be achieved through amputation or tumor resection followed by reconstruction (limb-salvage surgery). Prior to the 1970s, most primary bone sarcomas were treated with amputation.29,30 With the limited availability of adjuvant treatments and modern surgical techniques, this provided the patient with the best functional and clinical outcome. The 5-year survival rates were approximately 10% to 20%,30,31 with a vast majority of these patients succumbing to metastatic disease.29 With advancements in adjuvant treatment, there has been significant improvement in survival rates and a paradigm shift to limb-salvage surgery (LSS), with approximately 90% of primary bone sarcoma patients undergoing LSS.32 The following sections discuss the surgical approach to bone sarcomas, followed by a brief synopsis of the most common surgical procedures utilized in bone sarcoma limb-salvage reconstruction and their associated complications. Preoperative Considerations A multitude of factors help determine the optimal surgical procedure for the individual patient. Many of these are patient-specific factors discovered during the focused history and physical examinations. Various preexisting medical conditions (diabetes, autoimmune diseases, degenerative joint disease, vasculopathies, coagulopathies) may impact a patient’s ability to tolerate a major oncologic procedure or his or her ability to heal and rehabilitate. Behavioral (smoking) and/or psychological factors can also impact the patient’s ability to heal or comply with specific postoperative precautions or anticipated deficiencies. Many oncologic surgeries are extensive, and any bleeding or coagulopathy disorders should be elicited preoperatively and could require special treatment during invasive procedures and/or surgical treatment. All modifiable risk factors should be addressed prior to surgical intervention and medical conditions optimized in a timely fashion, so as to not delay treatment. Select patients with significant medical comorbid conditions and poor overall health may be better treated nonsurgically, particularly if they are at an unacceptable risk of mortality or morbidity with an extensive oncologic surgery. In certain instances, limb or osseous reconstruction may be associated with an unacceptable risk, and amputation or sarcoma excision without complex reconstruction is offered. Tumor-specific factors are also considered in developing a surgical plan for the sarcoma patient. Many pathologic factors impact the patient’s overall oncologic care, including the surgical plan. Specific tumor diagnoses and higher-grade tumors may necessitate a larger tumor margin or introduce alternative local control options. For example, extended intralesional curettage has become an acceptable treatment approach for low-grade chondrosarcomas, while high-grade tumors require wide resection.27 In unresectable Ewing sarcoma, radiation therapy is often implemented for local control owing to this tumor’s radiosensitivity.27 Many of the most critical factors that guide the planned surgery are interpreted from the preoperative imaging and biopsy results, making the quality and interpretation of these studies paramount. In many sarcoma centers, this is completed in a multidisciplinary sarcoma board to optimize the information gathered and to apply this information appropriately to treatment decisions.33 Critical tumor characteristics gleamed from radiographs include the location and size of the tumor, the mineralization pattern, and the osseous reaction to the tumor. MRI provides detailed information on the marrow extent of the tumor, including identification of skip metastasis, soft tissue extension, and the tumor relationship to (or involvement of) neurovascular structures. Interval MRIs often allow for estimation of neoadjuvant treatment response and can alter planned surgical resections.34 CT scan may be used an alternative when MRI is contraindicated or as an adjunct to radiographs in tumors involving complex bones (pelvis). En Bloc Resection The goal of sarcoma resection traditionally involves resection with a wide margin or en bloc resection.27 This is defined as a resection with a cuff of normal tissue. Ultraradical resections, which involve sacrificing vital structures to extend the margin, show no significant benefits from wide resection.27 The exact size of this wide margin has not been fully elicited from literature. In regard to high-grade, nonmetastatic osteosarcoma, Bertrand et al. found that the presence of a positive margin, compared to a negative margin >1 mm, was the only independent predictor of local recurrence after controlling for relevant confounding variables.35 Li et al. and Bispo et al. also investigated close margins, a margin 5 mm or less compared to >5 mm and margins <2 mm and >2 mm, respectively, and found no difference in likelihood of local recurrence in osteosarcoma patients.36,37 At this time point, no specific 57measurement could be borne out from the literature regarding margins, but local recurrence has been shown to be increased in osteosarcoma patients with positive margins.24 It appears that having a negative margin is an important factor in reducing local recurrence and increasing survival.27 When considering the goals of en bloc resection, it is important to consider that the majority of bone sarcomas present with soft tissue extension, requiring resection of associated muscle and soft tissues to achieve negative margins. Once the extent of resection is planned, the function of the residual limb can be estimated based on the residual functional tissue. If adequate margins require resection of critical structures, the anticipated function of the residual extremity will be impacted. While some critical structures are routinely reconstructed in an effort for limb salvage, the loss of multiple critical neurovascular structures often results in impaired neurologic or vascular function of the residual limb. Furthermore, large soft tissue defects may require secondary procedures, such as skin grafts and muscle flaps, to ensure a functional soft tissue envelope. The following sections provide overviews of the major surgical procedures employed in the treatment of bone sarcomas, commonly associated complications, and specific rehabilitation implications. Endoprosthetic Reconstruction Endoprosthetic reconstruction involves restoration of structural stability after skeletal resection through the use of a metal alloy implant and polyethylene at gliding surfaces. These are commonly used around periarticular or metaphyseal resections. Various endoprostheses are available for reconstruction throughout both upper and lower extremities. Most contemporary endoprostheses are modular and available in a range of sizes to restore a wide array of osseous defects. A distal femoral endoprosthetic reconstruction is seen in Figure 5.1. Depending on the surgical technique, many endoprosthetic reconstructions can allow for immediate weight bearing and mobilization. This is an advantage, as a subset of patients will be unable to ambulate in a non–weight-bearing fashion owing to comorbid conditions. For example, a patient with severe contralateral arthritis or poor overall condition may become bedridden in the absence of a construct that allows immediate weight bearing. Although it is a readily available method of reconstruction in limb-salvage surgery, endoprosthetic reconstruction is not without complications. Failures can occur from various methods, including soft tissue failures, aseptic loosening, structural failure, infection, and tumor progression. Henderson et al. performed a retrospective review of the modes of failure of large endoprosthetic reconstructions done after tumor resections.38 The authors identified a total of 6,533 patients who underwent large endoprosthetic reconstructions done after tumor resections; 2,174 reconstructions came from review of operative databases from five institutions and 4,359 reconstructions from review of the literature. Failure occurred in 27.6% of the reconstructions, with the most common mode of failure being aseptic loosening at 8.2%, followed by infection at 8.0%. Large endoprosthetic reconstructions are exposed to high stress at the bone–implant interface, given long lever arms and concentration of stress at the areas,39 which could contribute to this relatively high rate of failure. Also, patients undergoing orthopedic oncologic surgeries with reconstruction can be at higher risk for surgical site infection owing to extensive surgical dissection with longer operative times, adjuvant chemotherapy, and adjuvant radiation.40–42 Infection rate (8%) of large endoprosthetic reconstruction following tumor resection is notably higher than that for a primary total knee or total hip replacement for osteoarthritis, which have infection rates of 1.52% and 0.83%, respectively.43 Tumor progression was a mode of failure in only 4.9% of patients. Other modes of failure evaluated were soft tissue failure and structural failure, with failure rates of 1.5% and 4.9%, respectively. Henderson et al. also subdivided modes of failure by anatomic location. Proximal tibia endoprosthetic reconstructions had a failure rate of 39%, with infection being the most common mode of failure. Distal femur reconstructions had a failure rate of 26.8%, as compared to proximal femur reconstructions with a failure rate of 18.1%. Continued review of outcomes shows the trend that as the extent of resection and size of endoprosthesis increase, so does the rate of complications.44 FIGURE 5.1 Endoprosthetic reconstruction of distal femur. Pre- and postoperative images of a 14-year-old male with right distal femur osteosarcoma. (A) Preoperative radiograph showing a large distal femoral lesion with laminated periosteal reaction and anterior and cortical breakthrough. (B) Also evident is a large soft tissue mass, better seen on the sagittal T2-weighted MRI. (C) Postoperative radiograph after endoprosthetic reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree