The extent and the location of the primary tumour
The size of the breast
The density of the breast parenchyma and the grade of ptosis of the breast
The BMI and the body confrontation of the patient (very skinny, slim, normal, obese, very obese)
Previous breast surgeries
Tumour biology – especially when considering neoadjuvant treatment
Contraindications to radiotherapy
The age and comorbidities of the patient
Family history of the patient
Patient preference
18.3 Young Age: Family History
Young age is an independent risk factor for local recurrences [4]. According to a study conducted at the Dana-Farber Cancer Institute/Brigham, USA, between 1997 and 2006, the 5-year local recurrence rate was 5.0% for 23–46-year-old patients, whilst it was 2.2% for 47–54-year-old patients and just 0.6% for patients in the 55–63 years age group [20].
However, the risk of local recurrences has decreased significantly in recent years, and this decrease has been most striking among younger patients. According to a study based on the Eindhoven Cancer Registry, the 5-year local recurrence rates in patients 40 years or younger were 9.8% when treated in the period between 1988 and 1998, 5.9% when treated between 1999 and 2005 and just 3.35% when treated between 2006 and 2010 [21].
Young age is also associated with an increased risk of local recurrences in DCIS cases, despite the use of radiotherapy [11]. In particular, the risk of invasive local recurrence is higher [22]. Invasive local recurrences after breast conservation in DCIS, in turn, are associated with a poor prognosis [23, 24]. For these reasons, mastectomy should be discussed in young women with DCIS to minimize the risk of subsequent invasive local recurrence, in a similar way to discussions with high familial-risk women about risk-reducing mastectomy.
Young patients with a positive family history or even those with diagnosed BRCA gene mutations do not have an elevated risk of local recurrences, when compared with patients of a similar age [25]. Therefore, there is no reason to push these patients towards mastectomy. Instead, it is important to treat the cancer effectively and estimate the prognosis of the patient based on tumour stage and biology. Both ipsilateral and contralateral mastectomies are measures for secondary prevention in these patients. The need and the timing of mastectomy and contralateral mastectomy should be evaluated and discussed with the patient in the same way as with healthy high-risk women without cancer. However, unlike in healthy gene mutation carriers, the index cancer often determines the prognosis of the patient and therefore influences the benefit of the secondary prevention measures. However, in the case of DCIS, the prognosis is similarly excellent, and so prevention assumes much greater importance.
18.4 Multifocal and Multicentric Cancer
In many guidelines, multicentric tumours and sometimes even multifocal tumours are considered to be contraindications to breast conservation. However, there is not much high-quality evidence underpinning the safety of breast conservation in patients with multifocal or multicentric tumours. The largest series with the longest follow-up comes from Milan. During a median follow-up of more than 6 years, the 5-year local recurrence rate was 4.9% in the 421 patients with multifocal disease and 8.0% in 55 patients with multicentric tumours [26]. Smaller studies have reported 0–4.5% local recurrence rates during follow-up periods of between 3.5 and 4.6 years [27, 28]. These figures seem higher when compared to patients with unifocal tumours, but the local recurrence rates are also higher in patients with multifocal and multicentric cancers even when treated with mastectomy which may reflect disease biology rather than surgery type [29] (◘ Fig. 18.1).
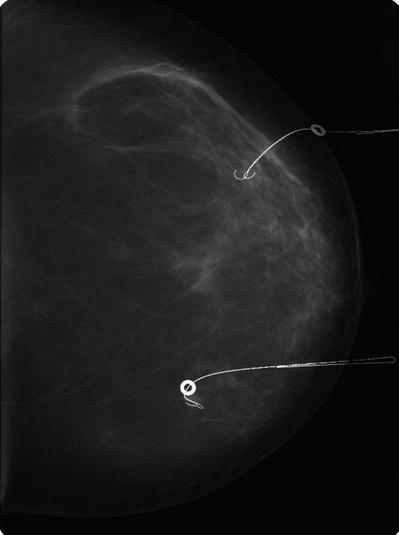

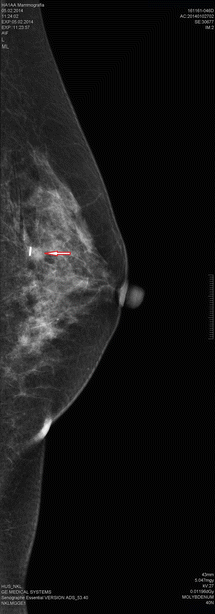
Fig. 18.1
Screen detected multicentric breast cancer in a 61-year-old woman. The small tumours were located at 3 and at 9 o’clock and were localized with two guidewires (2 mammographic projections). The 7 and 10 mm tumours represented invasive ductal cancer, and the resection margins were excellent. No recurrences have been observed during 5 years of follow-up
The level of evidence behind guidelines recommending mastectomy in patients with multifocal or even multicentric cancers is low, largely at the level of expert opinion, the lowest level in the hierarchy of evidence. Therefore, breast conservation may be considered in these patients provided they are aware of the academic uncertainty of this treatment and provided negative margins can be achieved without breast deformity. The use of MRI and an oncoplastic approach is helpful in these cases [30]. Individual decision-making is necessary, and each case should be discussed in the multidisciplinary team meeting and should also respect patient preference.
18.5 The Role of Radiotherapy
Breast radiotherapy is an essential part of breast-conserving treatment. According to the EBCTG meta-analysis published in The Lancet 2005, radiotherapy after breast conservation not only decreases the local recurrence rate but also improves survival [19].
Radiotherapy also substantially reduces the risk of local recurrences in elderly breast cancer patients; however, whilst in younger women this leads to a small survival advantage, in the elderly it does not. This difference is probably due to the shorter life expectancy of older women as the survival advantage of radiotherapy is not apparent until 15 years after treatment, by which time many older women will have died of other causes [31]. If an elderly patient is too frail for radiotherapy, breast radiotherapy can be omitted, if breast conservation is the patients’ preference. Endocrine therapy should be given in patients with endocrine-responsive tumours whether they have radiotherapy or not.
For some patients who live in remote areas, the requirement for daily travel to the radiotherapy centre for several weeks may be burdensome. For some this may steer them to choose mastectomy. However, intraoperative radiotherapy or other forms of brachytherapy may be given in selected, low-risk cases. The short-term outcome after intraoperative radiotherapy in selected patients has been excellent [32, 33], but longer follow-up is needed to establish the role of the intraoperative radiotherapy.
A history of previous radiotherapy to the thoracic area, for example, mantle radiotherapy for Hodgkin’s disease, may compromise the ability of a woman to have breast conservation as the previous radiotherapy fields may have overlapped with the tangential breast fields used in whole-breast radiotherapy after conservation. The fields of previous radiotherapy should be checked with a radiation oncologist before decision-making, to avoid an unnecessary mastectomy. When mastectomy is necessary, immediate breast reconstruction should be discussed with the patient.
18.6 Previous Breast Surgery
The patient may have undergone previous breast surgery, such as for benign diseases or more cosmetic procedures such as reduction mammoplasty or implant augmentation. These previous breast surgeries are not a contraindication to breast conservation. However, individual tailoring of surgery is needed and is sometimes challenging when previous surgical scars interfere with surgical planning for cancer surgery.
Breast conservation is feasible in patients who have undergone previous reduction mammoplasty, mastopexy, or breast resection of a benign breast lesion if the tumour size is not too large in relation with the size of the breast. However, the scars and the pedicle from a previous surgical procedure should be taken into account when planning the operation. The risk of fat necrosis and necrosis of the nipple areolar complex may be higher than usual, and this risk must be fully discussed with the patient.
Patients who have undergone previous implant augmentation may have unrealistically high expectations regarding the cosmetic outcome of cancer surgery. This relates to the fact that they often have a limited amount of the native breast tissue which may limit the feasibility of breast conservation, especially if the implant is removed. Moreover, if the implant is removed, the contralateral breast usually needs surgery for symmetry [34]. The placement of the scar may also limit the use of certain oncoplastic pedicles.
If the implant is not removed, there is up to a 65% risk of capsular contracture after breast-conserving surgery and radiotherapy, possibly leading to further surgeries, poor cosmesis and pain [34]. If the implant is sub-glandular, the tissue remaining after wide local excision may not be sufficient for implant coverage. In these cases, the sub-glandular implant can be changed to a smaller, sub-pectorally placed implant. Also in these cases surgery to the contralateral breast is often needed, to change the size and placement of the contralateral implant. When planning breast conservation in patients with a previous implant augmentation, all the aforementioned issues have to be considered and discussed with the patient. Implant choice is also important, especially if an expander implant is used as some integral ports may impact on radiotherapy delivery.
18.7 The Role of Breast MRI in Patient Selection for Breast Conservation
Breast MRI may reduce the need for reoperation due to positive resection margins in premenopausal patients [35] and in those with invasive lobular cancer [36]. However, breast MRI does not reduce the risk of local recurrences [37]. Moreover, the risk of local recurrence is similar in patients with invasive ductal carcinoma and invasive lobular carcinoma, even without MRI [3].
Breast MRI may lead to unnecessary mastectomies [36]. Therefore, all additional lesions detected by MRI should be biopsied before planning more extensive surgery, especially before recommending mastectomy to the patient instead of breast conservation. Consequently MRI may also delay surgery [38]. MRI is not helpful when planning surgery in patients with DCIS [39].
However, in patients undergoing neoadjuvant therapy, MRI is superior to breast ultrasound, mammography or clinical evaluation when evaluating the size of the residual disease in the breast [40]. Therefore, MRI is recommended in patients who are candidates for breast conservation after neoadjuvant treatment. A baseline scan is required, both for comparison with post-treatment scans to assess response but also to assess the pattern of disease before treatment to assess and likely success of NAC in facilitating conservation.
18.8 The Role of Oncoplastics
Good cosmesis is an important goal in breast conservation. When doing conventional wide local excision, breast deformity is likely when more than 20–30% of the breast tissue is removed [41]. Even a smaller volume excision may cause deformity, when the tumour is located either medially or caudally in the breast [41]. In these cases deformity can usually be avoided by using an oncoplastic approach. Oncoplastic breast surgery allows larger excisions whilst maintaining good cosmesis and thus also extends the indications for breast-conserving surgery.
18.9 Breast Symmetry
To achieve symmetry after oncoplastic breast conservation, surgery to the contralateral, healthy breast may be necessary. The need for, and timing of, symmetrization surgery should be discussed with the patient. Many patients are tolerant of even major degrees of asymmetry such as several cup sizes between the breasts, whilst minor asymmetry may annoy others. Asymmetry is not just about volume discrepancy but may relate to changes in the breast shape or degree of ptosis. Again patients vary in their tolerance, and whilst some women will be happy if they can achieve «in bra» symmetry, for others, perfect unclothed symmetry is desired. Correcting deformity is time consuming, and the outcome may not be as desired, even after several sessions of autologous fat grafting or a range of other techniques, and it is preferable to plan primary surgery to avoid these problems in the first place. These corrective procedures may also be more prone to morbidity due to the fact that the surgeon will be operating on irradiated tissues, leading to higher rates of wound breakdown, fat necrosis and capsule formation if implants are used. The contralateral breast may usually be successfully corrected for symmetry, in many cases with less risk, if the patient is prepared to accept a change in breast shape and size.
When operating on patients with cancer who are candidates for reduction mammoplasty due to macromastia, regardless of their cancer diagnosis, it is usually advisable to perform contralateral reduction at the same time as their cancer surgery to avoid gross size asymmetry. In other cases where breast size and/or ptosis is less marked, it is better to postpone the contralateral symmetrization procedures for 2–3 years. By this stage the treated breast will have fully recovered from radiotherapy and can be used as a template for the symmetrization procedure.
18.10 Other Important Aesthetic Issues
Complex oncoplastic surgery should not be used if deformity can be avoided without it. It is advisable to always select the simplest surgical technique to achieve the desired aesthetic outcome.
Another factor to consider when planning BCS is optimal scar placement. Scarring, especially if poorly sited, may be a cause of patient dissatisfaction. Good quality subcuticular suturing and a sympathetically placed scar (e.g. ideally avoiding the cleavage area) are important. Scars may be «hidden» in the inframammary fold or at the junction of the areolar and breast skin. Radial scar placement may contract and pull the nipple out of position and so peri-circumareolar scars may be better. Such scars also run in Langer’s lines and will tend to heal with less prominent scarring. Postoperative radiotherapy will make the scars in the treated breast less visible, but will have no impact on any scars in the contralateral breast, if symmetrization surgery is performed. This has to be discussed with the patient.
Complications like infection and skin or fat necrosis will destroy the aesthetic outcome of the breast after breast conservation, and these should be avoided, whenever possible. It is important to take into account comorbidities and the smoking history of the patient, which may increase the risk of wound complications.
The selection of surgical technique should take into account the consistency of the breast parenchyma. In dense breasts, any technique may be chosen. However, extensive undermining should be avoided in fatty breasts, because it often leads to fat necrosis [41]. In a patient with very fatty and fragile breast tissue, the classic reduction mammoplasty techniques may lead to fat necrosis. The more fatty and fragile the breast parenchyma is, the more simple and straightforward surgical technique should be.
It is not enough that the shape of the breasts and the symmetry are excellent. Also the size of the breasts after surgery should be large enough in relation to the body habitus of the patient. Very small breasts in an obese patient may lead to poor body image. Efforts should be made to establish the desired breast size of the patient and match this to the surgical outcome if possible.
18.11 The Role of Neoadjuvant Systemic Treatment
The size of the tumour may be just too large to allow breast conservation even with an oncoplastic approach. In these cases, the tumour can often be downsized by using primary systemic therapy, either chemotherapy or endocrine therapy. Careful patient selection is crucial: patients with multifocal or multicentric disease and those with extensive microcalcifications are not optimal candidates for this treatment option.
Although the response rate in general is good or even excellent (depending on the biological subtype of the disease), not all responders will achieve breast conservation. The response can be total or partial. If partial, the response may be concentric, but not sufficient. The response may also be honeycomb-like, so that the extent of the tumour is the same as before the treatment [42] (◘ Fig. 18.2).
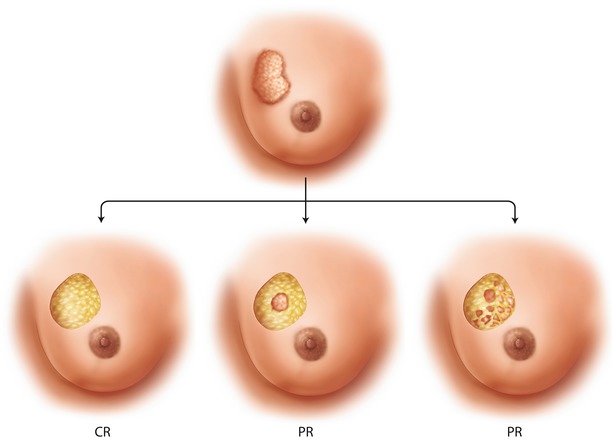

Fig. 18.2
The response to neoadjuvant chemotherapy may be complete, partial but concentric or partial and honeycomb-like. The latter case which is breast conservation is not feasible
As regards neoadjuvant chemotherapy to facilitate breast conservation, there should be a good oncological indication for chemotherapy in terms of disease prognosis and subtype. Patient should also be a good candidate for chemotherapy as regards to their age and comorbidities. It is also advisable to discuss the expected response and the probability that breast conservation will be possible after chemotherapy. Tumour biology influences the response rate. The response is best in triple negative and HER2-positive tumours, when compared with luminal-type tumours [43]. Patients with invasive lobular cancer often have multicentric or multifocal disease and tend to respond poorly to neoadjuvant chemotherapy, although for some of these patients the response may be sufficient to achieve breast conservation [44]. In patients with ER-positive tumours, breast conservation can also be attempted with neoadjuvant endocrine therapy.
The clinical response to neoadjuvant treatment can be complete tumour regression. Therefore, the tumour should be marked with a clip before starting neoadjuvant treatment. A radioactive seed may be used for this purpose or a simple metal clip. The radioisotope in the seed is I125, which has a half-life of 60 days. This has the advantage that the radioactivity remains for long enough that it can be used to permit gamma probe localization at surgery without an extra localization method, even after neoadjuvant chemotherapy [45].
When the aim of neoadjuvant treatment is breast conservation, the response should be monitored by breast imaging. MRI is the most accurate method to evaluate the size and the pattern of residual disease [40]. Breast ultrasound may also be used. Ideally the same imaging method should be used throughout when evaluating the response as switching modalities may misinterpret response.
18.12 Technical Considerations in Breast Conservation
18.12.1 Lumpectomy Only or Full-Thickness Wide Local Excision Including Overlying Skin and Underlying Pectoral Fascia
It is not necessary to perform full-thickness wide local excision in all patients. Lumpectomy including overlying skin and the underlying fascia is not necessary if the tumour is not located close to the skin or close to the fascia (◘ Fig. 18.3a). However, in these cases not only the radial margin but also the anterior or posterior margin or both have to be evaluated, and when positive, redo surgery should be considered. When the skin overlying the tumour is included, the anterior margin does not matter (◘ Fig. 18.3b). When the underlying fascia is included in the specimen, the posterior margin does not matter, unless the tumour is infiltrating the fascia or the underlying muscle (◘ Fig. 18.3c, d). The latter cases are readily recognized during surgery, and it is easy just to excise a circular piece of the underlying pectoral muscle to ensure a negative posterior margin.
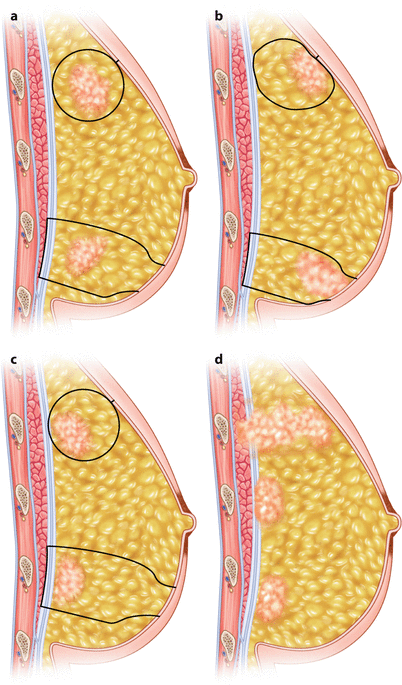

Fig. 18.3
a wide local excision can be either full-thickness type including both the overlying skin and underlying fascia. When the tumour is not located close to the skin or fascia, a full-thickness resection is not necessary, but in these case also anterior and posterior margins matter. b When the tumour is located adjacent to the skin, excising a slice of the overlying skin ensures anterior margin. c When the tumour is located adjacent to the pectoral fascia, excising the underlying fascia and overlying skin ensures posterior margin. d The tumour may infiltrate the underlying pectoral fascia or even the muscle. In this case, local excision of the underlying pectoral fascia and underlying muscle ensures posterior margin
Sometimes extensive excision of the breast skin is necessary for aesthetic purposes, to downsize the skin envelope during oncoplastic surgery or correct a degree of ptosis.
18.13 Impalpable Tumour Localization
With the widespread adoption of screening across Europe, a significant proportion of breast cancers are clinically occult/impalpable. It is technically challenging to excise impalpable cancers with negative margins and to do this without removing too much healthy breast tissue. There are a number of different techniques to facilitate specimen localization, and these are reviewed below.
18.13.1 The Guidewire
The gold standard in the localization of impalpable tumours has been the placement of a guidewire under ultrasound, stereotactic or even MRI control. Most often ultrasound guidance is used, because 95% of tumours are visible on breast ultrasound. The advantage of the guidewire is that the distance of the wire from the tumour can be readily evaluated by confirmation mammography (unless the lesion is mammographically occult). In cases with a large area of microcalcification, the area can be bracketed with 2–3 wires. Also multiple lesions can be marked using two or even three wires (◘ Fig. 18.1). The disadvantages include patient discomfort, migration or loss of the wire after placement and risk of puncture injuries to the surgical team and pathology laboratory staff. For technical reasons, the insertion point of the wire is sometimes remote from the tumour site, occasionally even in a different breast segment. This makes planning of the incision challenging, even when the tip of the wire is close to the tumour. Another issue is the logistics of coordinating wire insertion in the imaging department before surgery [46, 47].
18.13.2 Roll
Radioisotope localization of sentinel nodes led to an idea to localize not only the sentinel node but also the impalpable tumour with radioisotope. This technique is called radioguided occult lesion localization (ROLL) [48]. The technique can be performed in a number of ways. Two different isotopes may be used, one with small particles injected superficially to localize the sentinel nodes and another isotope with larger particles to the tumour site [48]. It is also possible to use a single peri- or intra-tumoural injection of radioisotope, both for tumour and sentinel node localization [49]. The disadvantage of the latter practice is less frequent sentinel node visualization, especially in elderly and obese patients with a tumour located deep in a large, fatty breast. The visualization of sentinel nodes is better after a superficial injection.
The advantage of ROLL over wire-guided localization is in patient comfort. There is no need for the patient to spend time with the discomfort and inconvenience of having a wire in situ whilst waiting for surgery, and also the isotope injection may be more comfortable when compared with wire placement. Also the placement of the incision is easier; it can be directed according to the point of maximum radioactivity. The presence of radioactivity in the resected specimen and in the resection cavity can be checked after resection using the handheld gamma probe to confirm localization. The logistical problems are similar as in guidewire placement due to a short half-life of the isotope. One possible problem is the lack of ability to confirm co-localization of the radioisotope and the tumour on post-placement mammography. Rarely, the liquid radioisotope may migrate in the breast, for example, along the injection channel, which may cause difficulties during surgery.
18.13.3 Radioactive Seed Localization
Localization of impalpable tumours with I125 radioactive seeds was introduced as early as 2001 [50], but the method did not gain popularity because of the introduction of ROLL. However, the method has been revisited and has recently gained increasing popularity. This procedure combines the advantages of ROLL and wire localization. The location of the seed in relation to the tumour can be readily evaluated by post-placement confirmation mammography (◘ Fig. 18.4), but the patient has the advantage that they have no need to spend time with a wire in situ before surgery. A large area of microcalcification may also be bracketed with 2–3 seeds. Moreover, the radioactivity is very focal, and there is minimal risk of migration after placement. Therefore, the resection can be directed even more accurately when compared with ROLL with liquid radioisotope, at least in theory. Also multiple lesions in the same breast can be marked separately which can be challenging and uncomfortable with wire placement techniques.