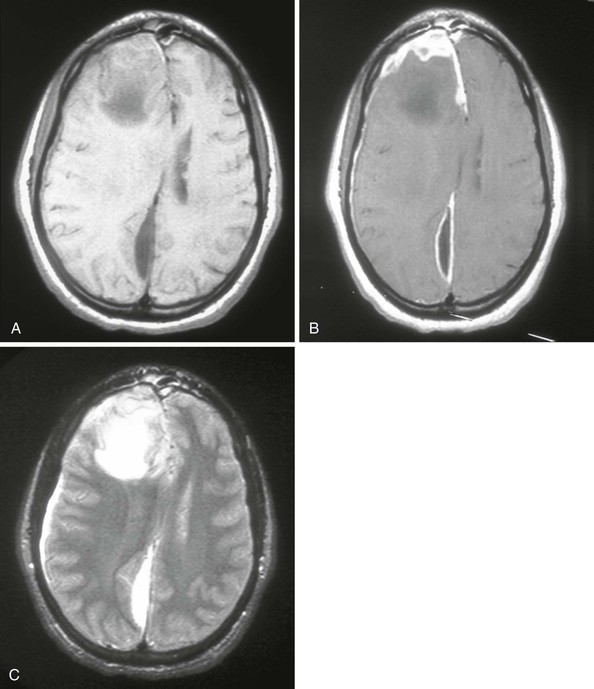
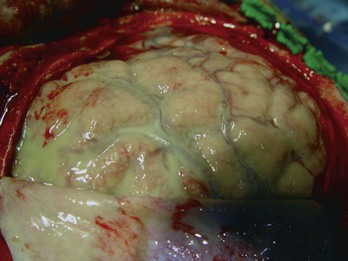
Allan R. Tunkel Subdural empyema refers to a collection of pus in the space between the dura and arachnoid. It accounts for 15% to 20% of all localized intracranial infections. Before the advent of antimicrobial therapy, the disease was essentially fatal (almost 100% mortality), but with current methods of diagnosis and treatment, mortality rates are approximately 10% to 20%.1–5 The most common conditions predisposing to cranial subdural empyema are otorhinologic infections, especially of the paranasal sinuses, which are affected in 40% to 80% of cases1,2,4–7; this percentage may even be higher in children.8–10 In contrast, in one 20-year review of 31 children with subdural empyema, only about 10% had a prior otorhinolaryngeal infection, with about 20% developing subdural empyema after head trauma or neurosurgery.11 The pathogenesis of cranial subdural empyema involves spread of the infection to the subdural space via valveless emissary veins in association with thrombophlebitis or via extension of osteomyelitis of the skull with accompanying epidural abscess. Once the infection reaches the subdural space, it can spread without interruption over the convexities of the brain. The mastoid and middle ear are the source in 10% to 20% of patients, especially in geographic areas where cases of otitis media are not treated promptly with antimicrobial therapy. Other predisposing conditions in patients with subdural empyema include skull trauma, neurosurgical procedures, and infection of a preexisting subdural hematoma.4,5,12–15 The infection is metastatic in about 5% of cases, principally from a pulmonary source. Rare predisposing factors include cranial traction devices, nasal surgery, ethmoidectomy, and nasal polypectomy.4 In one series of patients with infratentorial empyema,16 cases tended to cluster in the spring and summer months, with disease seen more commonly in males than females (65% vs. 35%). In infants with subdural empyema, meningitis is an important predisposing condition.17 Subdural empyema occurs in about 2% to 10% of infants with bacterial meningitis,5 presumably secondary to infection of an initially sterile subdural effusion. However, Haemophilus influenzae type b immunization has decreased the incidence of bacterial meningitis in infants and children (see Chapter 89), and cranial subdural empyema is now more commonly seen in teens and young adults.18 A number of bacterial species have been isolated in patients with cranial subdural empyema,3,4,5,6,19 including aerobic streptococci (25% to 45% of cases), staphylococci (10% to 15% of cases), aerobic gram-negative bacilli (3% to 10% of cases), and anaerobic streptococci and other anaerobes (33% to as much as 100% in some small series in which careful culturing was performed). Polymicrobial infections are common. These organisms make up the microbial flora that is frequently isolated from patients with chronic sinusitis or cranial abscess. Postoperative and post-traumatic infections are more commonly caused by staphylococci and aerobic gram-negative bacilli. Unusual pathogens include Salmonella species20,21 and Propionibacterium acnes.22–24 The latter microorganism has been isolated after trauma, neurosurgical procedures, or use of prosthetic material such as dural allografts, a material no longer used. In two series, 17%5 and 21%4 of samples taken from patients with cranial subdural empyema were sterile, although operative cultures have been reported to be sterile in 7% to 53% of cases.7 Cranial subdural empyema caused by Mycobacterium tuberculosis25,26 and Candida species27 has also been reported. Spinal subdural empyema is a rare condition that usually occurs secondary to metastatic infection from a distant site.28,29 The most frequent microbial isolate is Staphylococcus aureus, with streptococci, coagulase-negative staphylococci, and gram-negative bacilli found less frequently.30 The clinical presentation of cranial subdural empyema can be rapidly progressive, with symptoms and signs secondary to the presence of increased intracranial pressure, meningeal irritation, or focal cortical inflammation.1–5,7,30 This acute presentation of subdural empyema is seen most often in patients with contiguous spread of infection; the diagnosis should be considered in patients with acute bacterial sinusitis in combination with severe intractable headache, varying degrees of altered level of consciousness, focal neurologic deficits, signs of meningeal irritation, or a combination of these.31,32 Fever higher than 39° C is present in most cases. Headache, which may be localized to the infected sinus or ear, is a prominent complaint and becomes generalized as the infection progresses. Vomiting is common as intracranial pressure increases. Altered mental status (i.e., drowsiness and disorientation), which progresses to obtundation or coma if the infection is not treated, is seen in more than two thirds of cases. However, the triad of fever, headache, and altered mental status may be less commonly seen in children than adults.10 Focal neurologic signs appear in 24 to 48 hours and progress rapidly, with eventual involvement of the entire cerebral hemisphere. The most common focal signs are hemiparesis and hemiplegia; ocular palsies, dysphasia, homonymous hemianopsia, dilated pupils, and cerebellar signs have all been reported. However, in one study, no focal signs were observed in 41% of 699 patients with cranial subdural empyema.5 One third of patients in this series also presented with subgaleal abscesses, suggesting that the infection had spread from the calvarium across the dura. Seizures (either focal or generalized) are seen in 25% to 80% of cases.7 Signs of meningeal irritation are present in approximately 80% of patients, although fewer have either Kernig’s or Brudzinski’s sign. In untreated patients, there is rapid neurologic deterioration with signs of increased intracranial pressure and cerebral herniation, although papilledema develops in less than 50% of cases. This fulminant presentation, however, may not be seen in patients who have subdural empyema after cranial surgery or trauma, those who have received prior antimicrobial therapy, those with infected subdural hematomas, or those with infections metastatic to the subdural space. In one review of 55 patients with traumatic cranial subdural empyema,15 headache (84% of cases), fever (69% of cases), and neck stiffness (65% of cases) were the most common clinical features, and the mean time from initial trauma to presentation was 19 days (range, 4 to 60 days). In those patients with infratentorial empyema, clinical features are usually nonspecific with the triad of fever, headache, and vomiting the most common symptoms16; cerebellar signs, which could localize the lesion to the posterior fossa, were elicited in only 40% of cases. In subdural empyema of infancy (which results in patients with bacterial meningitis), persistent fever, declining neurologic status, seizures, or some combination of these is most frequently observed.18 The clinical presentation of spinal subdural empyema is usually one of radicular pain and symptoms of spinal cord compression, which may occur at multiple levels.29,30,33 The clinical presentation is difficult to distinguish from that of spinal epidural abscess, although tenderness on palpation is said to be a feature of epidural abscess and not subdural empyema (see later discussion). The diagnosis of cranial subdural empyema should be considered for any patient who presents with meningeal signs and a focal neurologic deficit.1–5 A lumbar puncture with cerebrospinal fluid (CSF) analysis is contraindicated because of the risk of cerebral herniation. In one large series,5 280 patients with cranial subdural empyema underwent lumbar puncture; 33 patients were thought to have experienced neurologic deterioration as a direct result of this procedure, with 3 unnecessary deaths. In patients in whom a lumbar puncture has been done, CSF findings have been nonspecific and have included an elevated opening pressure; neutrophilic pleocytosis (although in up to 40% of cases, there may be a mononuclear pleocytosis); and an increased CSF protein concentration.30 CSF Gram stain and cultures are negative unless the course is complicated by the presence of bacterial meningitis. Cranial radiographs may show evidence of concurrent sinusitis or osteomyelitis, but they are not useful in establishing the diagnosis of subdural empyema. Diagnostic procedures for cranial subdural empyema are either computed tomography (CT) with contrast or magnetic resonance imaging (MRI).1,7,29,30 The typical CT appearance is that of a crescentic or elliptical area of hypodensity below the cranial vault or adjacent to the falx cerebri; loculations may also be seen. Depending on the extent of disease, there may be an associated mass effect with displacement of midline structures. After the administration of intravenous contrast, a fine, intense line of enhancement can be visualized between the subdural collection and the cerebral cortex. MRI provides better clarity of morphologic detail and may detect the presence of a subdural empyema not seen on CT; it is particularly helpful in detecting subdural empyemas located at the base of the brain, along the falx cerebri, or in the posterior fossa. MRI can also differentiate extra-axial empyemas from most sterile effusions and subdural hematomas. Diffusion-weighted images and apparent diffusion coefficient images add to the diagnostic value of MRI; in patients with subdural empyema, there is consistently restricted diffusion (high signal) with corresponding low signal on apparent diffusion coefficient images.34 Based on these differences, MRI is now considered the diagnostic procedure of choice for cranial subdural empyema (Fig. 93-1). Both CT and MRI are also useful for demonstrating sinusitis and otitis. MRI is the diagnostic procedure of choice for spinal subdural empyema30,35 because it is better than CT in defining the extent of the lesion. Subdural empyema is a medical emergency, and its treatment optimally requires a combined medical and surgical approach. Surgery (i.e., drainage) is necessary because antimicrobial agents alone do not reliably sterilize the empyema. Cultures (aerobic and anaerobic) of purulent material are necessary to guide the use of specific antimicrobial agents, and surgical decompression is useful for controlling increased intracranial pressure. Once purulent material has been aspirated via craniotomy or burr hole placement, antimicrobial therapy should be initiated based on Gram stain results and on the likely predisposing factor that led to the development of the subdural empyema. If S. aureus is a suspected pathogen, vancomycin should be used empirically but changed to nafcillin if the organism is found to be methicillin susceptible and the patient is not allergic to penicillin. Linezolid has been successfully utilized in isolated cases of subdural empyema caused by streptococci36 and can be considered for patients with subdural empyema caused by gram-positive bacteria who fail conventional therapy. Metronidazole is recommended if anaerobes are suspected. For infection likely caused by aerobic gram-negative bacilli, empirical therapy with cefepime, ceftazidime, or meropenem is appropriate, pending microorganism identification and in vitro susceptibility testing. Metronidazole is not necessary for antianaerobic activity if meropenem is used. Dosing for these agents can be found in Chapter 92. Depending on the clinical response, parenteral antimicrobial therapy should be continued for 3 to 4 weeks after drainage, although there are no firm data to support a specific duration of antimicrobial therapy in patients with subdural empyema; longer periods of intravenous, and perhaps oral, therapy may be required if the patient has accompanying osteomyelitis. There have been anecdotal cases in which cranial subdural empyema was treated with antimicrobial therapy alone. This approach may be appropriate in patients with minimal or no impairment of consciousness, no major neurologic deficit, limited extension of the empyema without midline shift, and early improvement with antimicrobial therapy alone.4,18 However, these selected patients need careful clinical and radiographic monitoring and probably longer courses of antimicrobial therapy. The goals of surgical therapy in cranial subdural empyema are to achieve adequate decompression of the brain and to completely evacuate the empyema. The optimal surgical approach in patients with cranial subdural empyema (drainage via craniotomy or burr holes) is controversial.1,18,30,37 Some studies have demonstrated a lower mortality rate in patients who have undergone craniotomy, although it may be that a larger number of patients treated with burr hole drainage were more ill and had a greater surgical risk. Use of burr holes with irrigation may be more efficacious for drainage in the early stages of subdural empyema when the pus is liquid,38,39 allowing easier aspiration. If drainage is accomplished via burr holes, multiple burr holes may be necessary to allow extensive irrigation. When the pus becomes thickened and loculated as the disease progresses, these patients should undergo craniotomy. However, craniotomy may be essential for posterior fossa subdural empyema or if drainage is inadequate after burr holes. If craniotomy is performed, wide exposure is necessary to allow adequate exploration of all areas where the empyema is suspected (Fig. 93-2). The neurosurgeon may also elect to leave drains or catheters in the subdural space, although this may increase the subsequent risk of nosocomial infections. In one report,40 the efficacy of craniotomy versus CT-guided burr hole or craniectomy drainage was analyzed during the periods 1983 to 1987 (189 patients) and 1988 to 1997 (509 patients). At operation, the empyema collections were sometimes found to be more loculated, tenacious, and extensive than indicated by neuroimaging studies. Based on this experience, craniotomy became the preferred method of drainage for these authors beginning in 1988. A significant improvement in outcome was demonstrated between the 1983 to 1987 study period (good outcome in 71.4% of patients) and the 1988 to 1997 period (good outcome in 86.1%; P =.001). Analysis of the entire database (1983 through 1997) revealed that the mortality rates were higher for patients treated only with drainage via burr holes (23.3%) compared with those who underwent craniectomies (11.5%) or craniotomies (8.4%). Patients with multiple burr holes or craniectomy drainage not only required more frequent operations to drain recurrent or remaining pus but also exhibited higher mortality rates and poorer outcomes. The authors recommended limited procedures (i.e., burr hole drainage or craniectomy) only for patients with septic shock, patients with localized parafalcine collections, and children with subdural empyemas secondary to meningitis (because there is usually no brain swelling and the pus is thin). These data were confirmed in another study of 90 patients with intracranial subdural empyema in which patients with a cranial bone opening procedure had a better result in terms of clinical improvement and clearance of the empyema on CT41; reoperation was more frequent among patients who had undergone burr hole surgery. Regardless of the initial surgical approach, several studies have shown that a number of patients require reoperation; in one study, reoperation was required in half of those patients treated with burr hole drainage and one fifth of those initially treated with craniotomy.42 Surgical correction of the antecedent otorhinologic infection may also be required. In the modern era, survival rates among patients with cranial subdural empyema are greater than 90% for those who are awake and alert at presentation but less than 50% for those who present unresponsive to pain.30 Furthermore, 10% to 44% of patients may experience permanent neurologic deficits.4,18 Cranial subdural empyemas may also be complicated by septic venous thrombosis, localized cerebritis, and cerebral abscesses. In a recent review that included 65 patients with supratentorial empyema and 27 patients with infratentorial empyema,43 mortality rates were 10.8% and 3.7%, respectively; the lower mortality in those with infratentorial empyema supports the importance of early surgery, effective management of hydrocephalus, and adequate antimicrobial therapy.16 In patients with spinal subdural empyema, empirical antimicrobial therapy should be directed against staphylococci, streptococci, and aerobic gram-negative bacilli,30 with adjustments based on culture results. Laminectomy is also necessary for drainage of the infection. Epidural abscess refers to a localized collection of pus between the dura mater and the overlying skull or vertebral column. Because cranial epidural abscess can cross the cranial dura along emissary veins, an accompanying subdural empyema is often present.1 Therefore, the bacterial etiology and pathogenesis of cranial epidural abscess are usually identical to those described earlier for cranial subdural empyema, with the initial focus usually in the paranasal sinuses, middle ear, or mastoid cells; 60% to 90% of cases are associated with sinusitis and otitis, and complications are more common in children, adolescents, and young adults.44 Cranial epidural abscess may also occur after trauma, fetal scalp monitoring, halo pin penetration, and recent intracranial, transnasal, or transmastoid surgical procedures.14,17,44,45 About 0.2 to 2/10,000 hospitalized patients have spinal epidural abscess, with apparent increases in incidence likely from rises in the number of injection drug users and use of therapeutic spinal interventions.44 Spinal epidural abscess usually occurs secondary to hematogenous dissemination from foci elsewhere in the body to the epidural space or by extension from local infection.46–59 Most patients have one or more predisposing conditions, a spinal abnormality or intervention, or a local or systemic source of infection.57 Bacteremia may be an important predisposing factor because the incidence of spinal epidural abscess is increased in patients who use injection drugs60,61 and in those who have intravenous catheters or infective endocarditis. Diabetes mellitus also appears to be an important risk factor; it has been identified in up to 50% of patients.49,51,54,62 Other predisposing factors include immunosuppressive therapy, human immunodeficiency virus infection, malignancy, renal insufficiency, and alcoholism. Hematogenous spread occurs in 25% to 50% of cases, secondary to infections of the skin (e.g., furuncles, cellulitis, infected acne), urinary tract infections, periodontal abscesses, pharyngitis, pneumonia, or mastoiditis. Mild blunt spinal trauma (a history elicited from 15% to 35% of patients) may provide a devitalized site that is susceptible to transient bacteremia, although it is unclear whether this represents a true risk factor for the subsequent development of spinal epidural abscess. A primary source of infection is not identified in 20% to 40% of patients46–49,58 Infection of the epidural space has also been reported after penetrating injuries, extension of decubitus ulcers or paraspinal abscesses, back surgery, lumbar puncture, CT-guided needle biopsies, and administration of epidural anesthesia or analgesia.62–67 Many cranial epidural abscesses are polymicrobial in origin and include anaerobic gram-positive cocci, staphylococci, streptococci, and anaerobic gram-negative bacilli.44 Anaerobic organisms are identified in cultures from many sinusitis-associated intracranial epidural abscesses. The infecting microorganism in most (50% to 90%) of the patients with spinal epidural abscess is S. aureus46–59,62,68–70; the proportion of cases caused by a methicillin-resistant strain has increased in recent years,57 representing 41% of cases in one series.70 Other isolates include aerobic and anaerobic streptococci (8% to 17% of cases) and aerobic gram-negative bacilli (10% to 17% of cases), especially Escherichia coli and Pseudomonas aeruginosa; Pseudomonas spp. are commonly isolated from injection drug users.44 Recent case series have noted an increased incidence of aerobic gram-negative bacilli, streptococci, and anaerobes. In one recent series from Taiwan, 28.5% of 42 cases of adult spinal epidural abscess were caused by gram-negative bacilli.71 More than one microorganism is isolated in about 5% to 10% of cases.46,49,50,61 Isolation of coagulase-negative staphylococci is associated with spinal procedures such as placement of catheters for analgesia, glucocorticoid injections, and surgery.57 In addition to numerous case reports of patients with spinal epidural abscess caused by other bacteria, epidural infection can also be caused by Nocardia and Actinomyces spp.72–74 and M. tuberculosis75; M. tuberculosis is a more common cause of spinal epidural abscess in regions with rising numbers of immunocompromised patients.44 Fungi have also been isolated in patients with spinal epidural abscess.50,76–78 Cases of Exserohilum rostratum epidural abscess were reported in association with epidural or paraspinal glucocorticoid injections of preservative-free methylprednisolone from a single compounding pharmacy (see Chapter 270).79 The clinical presentation in patients with cranial epidural abscess may be insidious and overshadowed by the primary focus of infection (i.e., sinusitis or otitis media).1,18,50 Fever and headache are the usual complaints, but the patient may otherwise feel well (leading to a delay in diagnosis) unless the clinical course is complicated (e.g., by development of subdural empyema, brain abscess, or meningitis). This insidious presentation occurs because the dura is closely opposed to the inner surface of the cranium, so the abscess usually enlarges too slowly to produce the sudden onset of major neurologic deficits (in contrast to the presentation in patients with cranial subdural empyema) unless there is deeper intracranial extension. Eventually, focal neurologic signs and seizures (either focal or generalized) may develop. About 45% of patients also have periorbital cellulitis or frontal edema.44 In the untreated patient, papilledema and other signs of increased intracranial pressure develop as the abscess enlarges. A cranial epidural abscess near the petrous bone may result in Gradenigo’s syndrome, which is characterized by involvement of cranial nerves V and VI, with unilateral facial pain and weakness of the lateral rectus muscle. The clinical findings in patients with spinal epidural abscess may develop within hours to days (usually after hematogenous seeding), or the course may be more chronic, over weeks to months (usually in association with vertebral osteomyelitis or another contiguous focus of infection).46–58,80 The presentation in most patients with spinal epidural abscess progresses through the following four clinical stages: (1) backache and focal vertebral pain, with tenderness on examination; (2) nerve root pain, manifested by radiculopathy or paresthesias, or both; (3) spinal cord dysfunction, characterized by defects of motor, sensory, or sphincter function; and (4) paraplegia. Pain is the most consistent symptom (70% to 90% of cases) and is usually accompanied by local tenderness at the affected level. Fever is reported in 60% to 70% of patients, although it was noted in only 32% of patients in one study.51 The specific neurologic signs depend on the level of spinal cord involvement. Because the neurologic manifestations are usually reversible before complete paralysis occurs, emergency imaging studies and intervention are necessary if the diagnosis is being considered (see later discussion). Patients with tuberculous spinal epidural abscess have a more insidious course than those with nontuberculous spinal epidural abscess due to the slow proliferation of M. tuberculosis compared with the rapid proliferation of pathogens in nontuberculous disease.44
Subdural Empyema, Epidural Abscess, and Suppurative Intracranial Thrombophlebitis
Subdural Empyema
Epidemiology and Etiology
Clinical Features
Diagnosis
Management and Outcome
Epidural Abscess
Epidemiology and Etiology
Clinical Features
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Subdural Empyema, Epidural Abscess, and Suppurative Intracranial Thrombophlebitis
93