Abstract
The endometrium develops within the uterus, originating from the urogenital ridge early in development. This mucosal lining consists of epithelial and stromal cells invested with a specialized vasculature that matures at puberty and orchestrates cyclic menses while providing an immunoprotected site for implantation of the fetal allograft. As with other hormone responsive proliferative tissues, such as breast or prostate, the endometrium is prone to pathology. In this chapter we review the physiology of this tissue and explore the mechanisms of the normal and abnormal menstrual cycle. The mechanisms of implantation and menstruation are discussed along with the clinical approach to the evaluation of the endometrium in health and disease.
Keywords
Growth factors, cytokines, EGF, integrins, implantation, menstruation, decidualization, steroid receptors, STAT3, LIF, abnormal bleeding, adenomyosis inflammation, miRNAs, biomarkers, uterine receptivity, uterine transplantation
The fallopian tubes, endometrium and myometrium, and uterine cervix function in concert to receive gametes, facilitate fertilization, support embryo growth, and ultimately orchestrate the timely expulsion of the mature fetus. The preparation of a receptive reproductive tract is programmed by ovarian steroid hormones acting directly on their cognate receptors and indirectly through the intermediacy of various steroid-regulated growth factors, cytokines, and intracellular signaling molecules. This chapter describes the structural and biochemical changes in the endometrium during the normal menstrual cycle and pregnancy, its clinical evaluation, and the pathophysiology of some relevant disorders related to endometrial function (see Chapter 14 , Chapter 25 , Chapter 26 ).
The components of a receptive endometrium include the luminal epithelium, which undergoes apical surface specialization expressing cell adhesion molecules that permit adherence of the blastocyst; glandular epithelial cells, which secrete substances that support blastocyst development; and decidual cells and large granular lymphocytes that modulate trophoblast function and blood vessels, regenerated cyclically via neoangiogenesis. The interregulation of these components is accomplished by the secretion and action of growth factors, growth factor-binding proteins, angiogenic factors, and cytokines, a stromal extracellular matrix that facilitates trophoblast invasion. The combined actions of the paracrine and endocrine factors, together with the extracellular matrix, promote trophoblast proliferation and penetration of the endometrium, while controlling excessive invasion.
Innate and adaptive immune functions, under the regulation of steroid hormones, collectively defend the reproductive tract environment but are also modulated during pregnancy to allow the fetal semiallograft to be accepted by the maternal host. The vascular system nourishes the endometrium in the initial receptive phase and is subsequently remodeled by invading trophoblasts to establish the placental blood supply. The coordinated contractile activity of the myometrium promotes sperm migration in a cycle of conception, while also facilitating embryo transport prior to attachment.
In the absence of conception, the endometrium is shed through a well-controlled inflammatory-like reaction involving matrix metalloproteinases (MMPs), inflammatory cytokines, the production of vasoactive substances, and uterine contractions that promote remodeling, hemostasis, and the expulsion of the spent endometrial lining. Compartmentalized and specialized endometrial cells at the menstrual interface provide remarkable regenerative capacity, ensuring that a new intact luminal endometrial surface is prepared for the next round of oocyte release and potential fertilization.
Structure and Function
- ◆
The primary function of the endometrium is to provide an immunoprivileged site for embryo implantation and to provide a nurturing environment for the fetus during pregnancy.
- ◆
The cyclic differentiation of the endometrium depends on the actions of steroid hormones that act through specific downstream mechanisms involving complex molecular signaling.
- ◆
The endometrium undergoes repetitive episodes of proliferation, secretion, and menstruation, up to 400 times during a woman’s life, without apparent signs of aging.
- ◆
Embryo implantation requires a complex series of endometrial changes that are considered essential for the establishment of endometrial receptivity.
- ◆
Inflammatory conditions in the endometrium result in a phenomenon known as progesterone resistance, which may be involved in infertility and pregnancy loss.
- ◆
Uterine transplantation has been successfully established, but long-term adverse consequences of immunosuppression and rejection have proven challenging.
Ontogeny of the Uterus
The female reproductive tract is derived from the urogenital ridge, which, during week 6 of gestation, gives rise to paired mesodermal, paramesonephric tubes (the müllerian ducts) formed from longitudinal invaginations of the coelomic epithelium (see Chapter 16 ). The distal ends become the fallopian tubes, with the caudal ends fusing by week 10 of gestation to form the primordial uterus and upper portion of the vagina. A thin septum remaining after fusion is eventually resorbed, yielding a single cavity of the uterus and vagina. The primordial uterus is initially lined by a simple cuboidal epithelium that subsequently becomes columnar and pseudostratified. Beneath this epithelium lies a condensed mesenchyme that yields the endometrial stroma and the surrounding myometrium. Glandular epithelium buds from the luminal epithelium, invaginating into the stroma, as demonstrated in animal models. By week 22 of gestation, the uterus has taken on the structure of the adult organ. Glandular secretory activity, glycogen accumulation, and stromal edema are evident by week 32 under the influence of placental-derived steroid hormones. After delivery, with the precipitous fall in placental estrogens and progesterone, the endometrium regresses to an atrophic state, containing a few small-caliber glands and a poorly vascularized stroma.
Role of the Wnt Family and Homeobox Genes
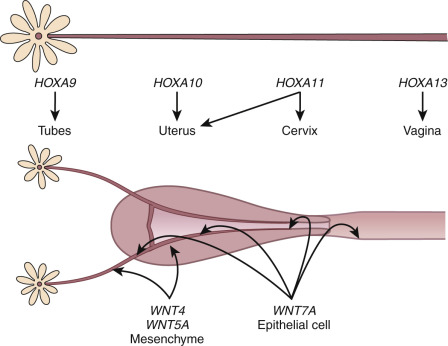
The embryonic events described previously are driven, in large measure by the secreted ligands of the wingless-type MMTV integration site (Wnt) family (WNT4, WNT5A, WNT7A) and transcriptional regulators of the homeobox (HOX) gene family ( Fig. 9.1 ). This morphogenetic program can only be played out in the absence of anti-müllerian hormone (AMH), a member of the transforming growth factor β family made by the Sertoli cells of the fetal testes. In the absence of testosterone and AMH, the müllerian ducts elongate and develop into the fallopian tubes, uterus, cervix, and upper part of the vagina. The elongation phase of the müllerian ducts requires a number of factors. Given their common embryonic origin, early development in the mouse of the kidneys, ureters, and reproductive tract are tightly linked and involve other specific genes, including Pax2, Lim1, Emx2 , as well as the members of the Wnt and abdominal-B HOXA families of genes. Lim1 encodes a transcription factor that along with PAX2 is essential for urogenital tract development. Lim1 null mice lack uteri and oviducts. Pax2 null mice lack kidneys, ureters, and genital tracts. Caudal elongation of the paramesonephric duct is absent. EMX2 is another transcription factor of the homeodomain gene family that appears to be essential for urogenital tract development. EMX2 is highly expressed in the adult uterus, and its expression is correlated with cell proliferation and appears to be inhibited by the HOX gene, HOXA10 . There is decreased expression of PAX2 and LIM1, and mesenchymal segmental polarity gene product, WNT4, is also absent in mice lacking EMX2, suggesting the essential role for this transcription factor.
The study of mice with targeted inactivation of Wnt genes revealed the importance of these signaling molecules in the development of the reproductive tract. The müllerian ducts are absent in female mice lacking Wnt4 , a gene expressed in the mesenchyme. Moreover, female mice lacking WNT4 are partially sex-reversed due to the retention of the wolffian ducts. Cases of Wnt4 null mutations associated with müllerian duct regression and a phenotype, including hyperandrogenemia, resembling that of the Wnt4 knockout mouse, have been reported in women. There is also a proposed role for WNT4 in postnatal uterine function, including progesterone signaling.
WNT9B is expressed in the wolffian duct epithelium and is necessary for müllerian duct extension. Mutations in the WNT9B gene have been found in women with Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. These müllerian defects have been reported to be associated with other gene defects, including PBX1, RBM8A, and TBX6 mutations. Other genes have been implicated as well, based on balanced translocation studies and breakpoint mapping.
Deficiency of Wnt5a , a gene expressed in the genital tubercle and genital tract mesenchyme, results in mice with stunted genital tubercles and the absence of external genitalia. WNT7A expression gives rise to the luminal and glandular epithelium of the fallopian tubes and uterus, while WNT5 is associated with stromal cells. WNT7A is involved in paracrine signaling to the endometrial mesenchyme. Mesenchymal β-catenin appears to be the essential downstream effector of the WNT7A pathway, and mediates its effects on the oviduct and proper development of the uterus. Although WNT7A mutation s have not been found in women with müllerian anomalies, mice lacking Wnt7a develop an oviduct that is not clearly demarcated from the upper uterine horn, and the uterus develops cellular characteristics that are similar to the vagina (including a stratified epithelium without uterine glands), and the uterine smooth muscle is disorganized. Postnatal expression of HOXA10 and HOXA11 in the uterus is also lost. Mesenchymal β-catenin appears to be the essential downstream effector of the Wnt7a pathway and mediates its effects on the oviduct and proper development of the uterus. The Wnt family of genes, including receptors and downstream signaling molecules, are also expressed in a regulated fashion in the adult reproductive tract, indicating that they have additional roles beyond those involved in early morphogenetic events, including the regulation of steroid hormone action in adult tissues (discussed in subsequent chapter text).
The HOX genes encode an evolutionarily conserved family of transcription factors that contain a signature 60 amino acid deoxynucleic acid (DNA)–binding homeodomain. They play critical roles in organizing cells along the anterior-posterior axis and directing them to select a particular pathway of development. Mammalian HOX genes are arranged in four different clusters, designated A through D, with each cluster organized in a linear arrangement that parallels the order of expression along the anterior–posterior body axis. The expression of HOXA genes in the human and mouse reproductive tract is conserved, with HOXA9 being expressed in the fallopian tubes, HOXA10 and HOXA11 in the uterus, HOXA11 in the cervix, and HOXA13 in the upper vagina. Although there is a consistent regional distribution of HOX gene expression along the reproductive tract, there is evidence for some functional redundancy among the adjacent genes. Like the WNT genes, the HOXA genes are also expressed in the adult uterus, and their expression is under estrogen and progesterone regulation.
The importance of the HOX gene family in reproductive tract function has been demonstrated through targeted deletions in specific HOXA genes. Another significant discovery was that hand–foot–genital syndrome and Guttmacher syndrome, autosomal dominant conditions that affect bones in the hands and feet and cause reproductive tract abnormalities (including bicornuate uterus), are caused by mutations in the HOXA13 gene. However, to date, mutations in HOXA7 to HOXA13 and HOX gene cofactor pre-B-cell leukemia homeobox1 (PBX1) have yet to be found in subjects with congenital absence of the uterus and vagina. HOXA10 and HOXA11 have both been implicated in the process of implantation. Mice with targeted deletions in the HOXA10 and HOXA11 genes have subtle abnormalities in uterine morphology, including transformation of the upper uterine segment into an oviduct-like histology ( HOXA10 mutants); reduced endometrial stromal development and expression of leukemia inhibitory factor (LIF) are seen in HOXA11 mutants. Notably, both HOXA10 and HOXA11 nullizygous females are infertile due to a uterine factor, implicating these genes in the implantation process in the adult. Mice lacking H6 homeobox 3 (Emx3) , another HOX domain gene product, are also infertile due to an implantation defect associated with perturbations in WNT and LIF gene expression.
Müllerian anomalies represent a complex and rare collection of developmental defects occurring in 5% of the general population. Depending on the stage of development at which they occur, these defects in the reproductive tract can be mild (including a uterine septum) or severe, with complete absence of the cervix, uterus, and fallopian tubes. These can be associated with infertility, endometriosis, and miscarriage, but also can require surgical correction and are often discovered at the time of puberty, if not before. Given the close developmental interaction between the müllerian and urinary system, it is not surprising that combined renal and müllerian duct agenesis occur. The patterns and genetics of müllerian anomalies provide further insight into the embryology of the reproductive tract morphogenesis.
Steroid Action in the Endometrium
Many, but not all, of the uterine responses to steroid hormones are mediated by specific intracellular cognate receptors (see Chapter 5 ). These nuclear receptors serve as transcription factors undergoing striking spatial and temporal changes in expression during the menstrual cycle. The endometrial response to steroid hormones is determined by the amount of bioavailable hormones, which is influenced by hormone production rates as well as local steroid metabolism; the repertoire of steroid receptors, coactivators, and corepressors expressed; and the action of growth factors and cytokines that modulate the action of steroid hormone receptors. Membrane-bound receptors with specific recognition of steroid receptors also coordinate the paracrine, autocrine, and juxtacrine cellular mechanisms, and provide an explanation for rapid effects of steroid hormones.
Rapid effects of estradiol are mediated by at least two distinct receptors, a membrane associated form of ERα and a recently described integral membrane receptor, G protein coupled estrogen receptor (GPER), previously known as G protein coupled receptor 30 (GPR30). GPER has been characterized as an estrogen receptor and subsequent development of a GPER-specific agonist, G-1, and antagonist, G-15, have revealed important functions of this nonclassical estrogen receptor in multiple physiologic and pathophysiologic processes. In the endometrium, this receptor is present in the endometrial epithelium during the late proliferative phase and transitions to the stroma and decidua in the latter stages of the menstrual cycle and pregnancy, but may be dysregulated in endometriosis. G-1 has been shown to induce cell cycle arrest, and thereby has some potential value in proliferative diseases such as endometriosis. GPER may also function as an aldosterone receptor, and though a mechanism conferring steroid specificity remains unclear, it may involve a coreceptor such as RAMP-3.
Estradiol is the primary trophic hormone for the uterus, mediating uterine growth through ERα, whose concentrations are highest during the proliferative phase of the cycle and decline after ovulation in response to rising progesterone ( Fig. 9.2 ). Immunohistochemical studies demonstrate estrogen receptors in the nuclei of epithelial, stromal, and myometrial cells during the proliferative phase, with the epithelial cell staining being most prominent. After progesterone levels rise in the luteal phase, estrogen receptor staining is restricted to the deep basal glands and vascular smooth muscle. In situ hybridization studies demonstrate that transcripts for both ERα and ERβ are expressed in the epithelial, stromal, and smooth muscle cells at all stages of the cycle.
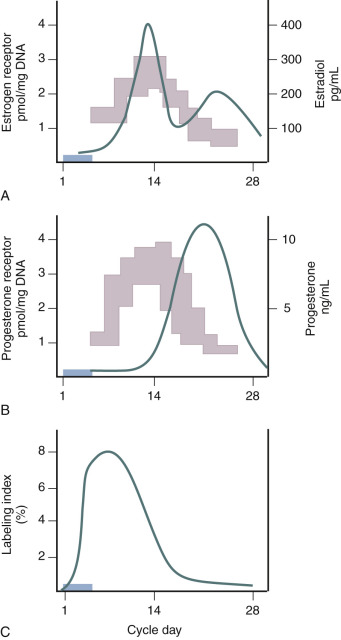
Is there a threshold dose of estrogen required to elicit a uterine growth response? Key and Pike proposed a threshold estrogen level of approximately 50 to 100 pg/mL, at which point endometrial proliferation is triggered and above which no further stimulation of endometrial proliferation occurs. This estimation was made on the basis of comparing endometrial proliferation assessed through ex vivo thymidine incorporation into endometrial explants from different stages of the menstrual cycle with corresponding estradiol levels at the different days of sampling. This hypothesis finds relevance in postmenopausal estrogen therapy where the effects of estrogen on bone, cardiovascular, and endometrial function may present differential risk/benefit profiles.
The decline in ERα at the time of implantation appears to be physiologically important and is a common finding across many species. Failure to downregulate ERα indicates an imbalance in regulatory mechanisms of steroid hormone interactions, and is associated with progesterone resistance, endometriosis, and infertility. The mechanism of this downregulation is complex, involving paracrine interactions through progesterone signaling that is discussed in more detail later in this chapter.
ERβ is expressed throughout the body expressed in almost all tissues. Despite a negligible effect on implantation in the knock out mouse, ERβ does appear to have importance in endometrial function. Like ERα, ERβ does appear to be upregulated by estrogen and downregulated by progesterone. Furthermore, in the ERβ knockout mouse, PGR levels rose, suggesting a suppressive action of ERβ on this receptor, and may also modulate ERα. In ERα depleted mice, treatment with estrogen blocked proliferation, suggesting an antiestrogenic effect and causing epithelial apoptosis through ERβ. In the human endometrium, ERβ has been reported to be expressed in both glands and stroma, but some suggest it is present exclusively in the vascular compartment. Relative overexpression of ERβ in ectopic endometrium of women with endometriosis has also been reported. Paradoxically, in humans, this increase in ERβ noted in endometriosis has been shown to stimulate progression of disease.
A variant of ERβ, ERβcx/β2, formed by alternative splicing of the eighth exon of the “classical” ERβ (ERβ1) transcript, has been described with distinct ligand and coactivator affinity. These isoforms have different selectivity for ligands and affinities coactivators. The patterns of ERβ1 and ERβ2 protein expression in the endometrium differ: ERβ1 expression appears to be more intense than ERβ2; ERβ1 protein levels are unchanged throughout the menstrual cycle, while ERβ2 levels decline in the glands of the functionalis layer but not the basalis layer in the midsecretory phase. The physiologic significance of these ERβ isoforms in the endometrium remains to be determined.
Other members of the estrogen receptor family include estrogen receptor–related α (ERRα) and β (ERRβ), orphan receptors, with homology to the classical ERα. ERRα and its coactivator peroxisome proliferator-activated receptor gamma (PPAR-γ) coactivator-1 α were found to show dramatically increased expression in the decidua, and stimulates metabolic pathways for energy generation, perhaps in preparation for implantation. ERRβ is seen throughout the endometrium during the menstrual cycle, including uterine natural killer (uNK) cells, but a precise role for this receptor has yet to be determined in the endometrium.
Progesterone antagonizes actions of estrogen in the endometrium and promotes glandular and stromal differentiation via progesterone receptors (PRs). The antagonism of the uterotropic actions of estradiol involves a complex series of events, including alterations in estrogen receptor expression, inhibition of estrogen-induced translocation of the cell-cycle regulators, and induction of enzymes that catabolize estradiol. All of these effects are mediated through specific cognate nuclear receptors that are induced by estrogen and modulate downstream events in a paracrine fashion between the epithelial and stromal compartments.
There are multiple isoforms of the PR, but the majority of progesterone actions are served by the activities of either PR-A or PR-B. Both arise from a common PR gene. Each isoform is expressed differentially in the endometrium, where the larger PR-B is a strong transcriptional activator of endometrial genes, while PR-A, 164 amino acid shorter, inhibits both PR-B and other steroid receptors, including estrogen receptor. The PR-A form predominates in the stroma, while the PR-B is more abundant in epithelial phase of the early secretory phase of the cycle. Both PR-A and PR-B act as transcription factors, interacting with specific gene promoters and interacting with coregulators. PR-B also has nongenomic mechanisms of action, able to interact with Src tyrosine kinases in the cytoplasm, which independently modify gene expression. While generally considered to have opposing functions, PR-A is essential for stromal decidualization, and PR-B is downregulated in the epithelial compartment at the time of pregnancy. Indeed, persistence of epithelial PR is thought to be a sign of defective endometrial receptivity and may signify progesterone resistance.
PR isoforms A and B are both present in the epithelial and stromal compartment of the proliferative and early secretory endometrium. Progesterone through PR serves to inhibit proliferation by estrogen, primarily by downregulation of the estrogen receptor, but also through induction of estradiol degrading enzymes. Unlike its antiproliferative effects in the glandular epithelium, stromal PR stimulates proliferation through activation of the MAPK/AKT pathway.
Multiple other derived isoforms of PR are thought to be present ( Fig. 9.3 ). In addition to PR-A and PR-B, there is a highly truncated isoform PR-C, identified in the T47D breast cancer cell line. While still able to dimerize with PR-A or PR-B, PR-C may be an inhibitory factor, unable to interact with gene promoters. A role in labor for PR-C has been proposed.
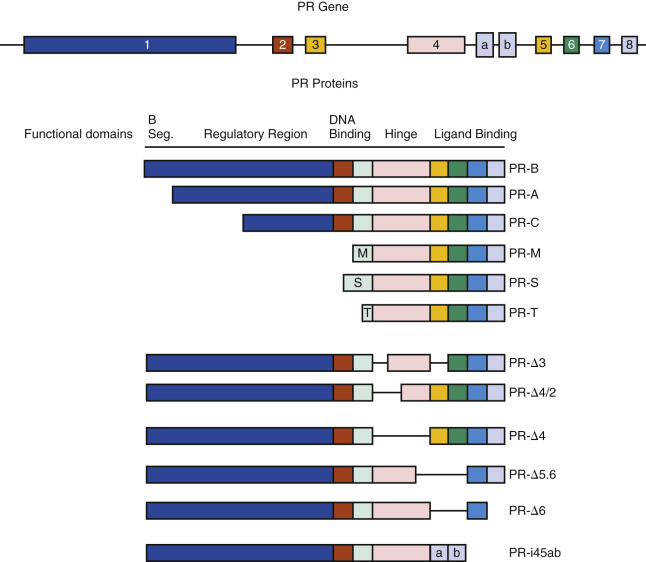
Classical endometrial PRs peak at the time of ovulation, localized to both epithelial and stromal cells, then decline. The rise in PRs is in response to estradiol, while the decline after ovulation is caused by elevated luteal phase progesterone that downregulates its own receptor. By 4 days after ovulation, PR staining of the epithelial cells declines markedly in the epithelial compartment and remains weak or absent during the remainder of the secretory phase. In contrast, PR expression in stromal cells remains strong throughout the menstrual cycle and into pregnancy, should it occur. In general, the PR-A form predominates in the stroma, while the PR-B is more abundant than the A form in the epithelial phase of the secretory phase of the cycle. PRs have not been detected in vascular endothelial cells or vascular smooth muscle, but they are abundant in perivascular stroma. Consequently, the effects of progesterone or its withdrawal on the vasculature are likely indirect or perhaps via membrane PRs.
The significance of different ratios of these two major PR forms, with respect to regulation of gene expression in the human uterus, is increasingly being studied. Clues are being provided from the study of mice with targeted deletion of the receptor isoforms. The uteri of mice lacking both the A and B form of the PR are hyperplastic and contain inflammatory infiltrates. Hyperplasia reflects the lack of antagonism to the uterotropic actions of estradiol. Selective targeting of the A form of the PR revealed that it is essential for progesterone-mediated actions on implantation and the decidual response. However, the examination of genes associated with the window of uterine receptivity that are known to be regulated by progesterone indicated that the A form controls expression of only a subset, while others appear to be under the control PR-B. Ablation of PR-A in mice uncovered an unexpected role for the B form of the receptor in inducing epithelial proliferation. Administration of estradiol to PR-A receptor–deficient mice resulted in uterine hyperplasia, but the combination of estradiol and progesterone resulted in even greater hyperplasia. It thus appears that PR A antagonizes the uterotropic responses mediated by both ERα and PR B. With regard to implantation and decidualization, however, when PR-A null mice are examined, they are similar in their defects to mice that lack both PR-A and PR-B subunits, while PR-B knockout mice (PRBKO) were fertile. Clinically, reductions in PR-B have been associated with proliferative states such as endometriosis, supporting this paradigm of counterregulatory mechanisms involving PR isoforms. In addition, like estrogen, nongenomic actions of progesterone appear important in the reproductive tract as well.
In addition to steroid hormone receptors, coactivators and chaperone proteins have an important impact on the uterine response to estrogens and progestogens. The uterine expression of p160 coactivators, steroid receptor coactivator-1 (SRC1), steroid receptor coactivator-2 (SRC2), and steroid receptor coactivator-3 (SRC3) has been examined. Decidualization of the endometrium does not occur without SRC2. SRC3 levels increased in the glandular epithelium in the late secretory phase, whereas SRC1 and SRC2 expression did not change. SRC3 has been linked to endometrial hyperplasia and in women with polycystic ovary syndrome (PCOS), SRC2 and 3, along with ERα, were elevated in stroma and glandular epithelium, demonstrating that an abnormal endocrine milieu can alter coactivator levels, which could in turn result in endometrial dysfunction.
Steroid Hormone Metabolism in the Endometrium
The activity of steroid hormone in the endometrium is determined in part by the modulating effects of uterine enzymes that actively catabolize steroid hormones ( Fig. 9.4 ; see Chapter 4 ). These enzymes that carry out transformations of steroid hormones are subject to regulation during the menstrual cycle. Estradiol taken up from the plasma can be converted into estrone by the action of 17β-hydroxysteroid dehydrogenases (17β-HSD), or converted to sulfated conjugates by estrogen sulfotransferase. Three different forms of 17β-HSD capable of oxidizing estradiol to estrone have been detected in primate endometrium: the type 2, type 4, and type 8 enzymes. Type 2 and type 8 enzymes are associated with microsomes; type 4 enzyme is in peroxisomes. Type 2 and type 4 enzymes use the oxidized form of nicotinamide adenine dinucleotide as a cofactor and are predominantly localized to glandular epithelium in the secretory phase. The endometrial type 2 enzyme shows the greatest change in expression during the cycle, peaking in the secretory phase. The type 8 enzyme appears to be constitutively expressed. Progesterone enhances the conversion of estradiol to estrone in endometrial cells by inducing expression of the type 2 17β-HSD and, to a much lesser degree, the type 4 enzyme. Progesterone also increases endometrial estradiol sulfation by increasing estrogen sulfotransferase activity. Thus progesterone activates enzymatic pathways that inactivate estradiol. However, there are regional differences in the balance of systems removing and restoring estradiol in the uterus. For example, estrogen sulfatase is detected only in the glandular epithelium of the basalis, where it may function to increase the level of estradiol from estradiol sulfate. The human uterus does not normally have the capacity to produce significant amounts of estrogen locally (either de novo or from circulating prohormones), but endometrial cancers, endometriotic lesions, and to a lesser extent, eutopic endometrium of women with endometriosis aberrantly express components of the steroidogenic machinery (P450 arom ) that endows the tissue with the capacity to synthesize estrogens from circulating adrenal or ovarian androgens or by de novo synthesis. Endometriosis lesions have been found to express StAR, the cholesterol side-chain cleavage enzyme, P450c17, aromatase, and type 1 17β-HSD in stromal cells (see Chapter 25 ). Estradiol produced in these lesions can enhance production of prostaglandin E2, which in turn stimulates transcription of the aromatase gene, resulting in a feedforward mechanism for increasing local estrogen levels. In addition, endometriotic lesions do not express the progesterone-regulated type 2 17β-HSD that converts estradiol to estrone, which effectively increases the bioavailability of estradiol.
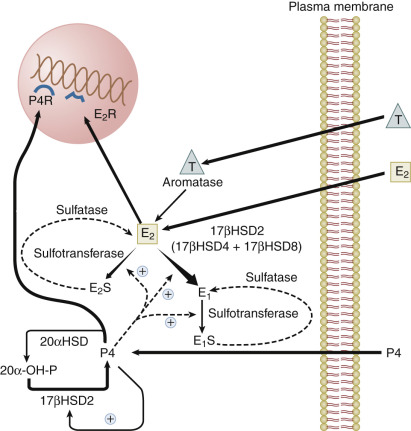
Progesterone is catabolized in the uterus into inactive 20α-hydroxyprogesterone by 20α-HSDs. The type 2 17β-HSD, which is increased in the secretory phase, is also a 20α-hydroxysteroid oxidase that converts 20α-hydroxyprogesterone back into progesterone. The induction of the type 2 17β-HSD by progesterone in the secretory phase therefore contributes not only to the catabolism of estradiol, but also to the maintenance of endometrial progesterone levels.
Paracrine Actions of Steroid Hormones in the Endometrium
Based on the pioneering work of Cunha, it now appears that most of the effects of estrogen and progesterone on epithelial and stromal proliferation and differentiation are indirect, involving the activation of downstream modulators in the stroma, which produces paracrine substances that act back on the epithelium. Estrogen acting through the stroma promotes DNA synthesis in the epithelium; under the influence of progesterone, the epithelium produces substances that affect response of the underlying stroma and the epithelial cells to estrogen.
These indirect actions of estradiol on epithelial proliferation have been demonstrated in elegant reconstitution and grafting experiments employing stroma and epithelium from wild-type and estrogen receptor knockout mice. Epithelial cell proliferation does not occur when the stroma from an ERα knockout (ERKO) mouse uterus is paired with epithelium from a wild-type uterus. Conversely, epithelial cell DNA synthesis in response to estrogen occurs when wild-type stroma is paired with epithelium from ERKO mice. Studies on human endometrial cells in culture are consistent with the mouse studies. Estradiol increases epithelial cell proliferation when cocultured with stroma, but it does not increase proliferation in epithelial cells cultured in the absence of stromal cells.
What is the estrogen-stimulated signal from the stroma that promotes epithelial cell proliferation? Candidates include growth factors under the transcriptional control of the estradiol receptor, including insulin-like growth factor-1 (IGF-1), transforming growth factor α (TGF-α), and epidermal growth factor (EGF). Alternatively, estradiol might suppress production of stromal factors that restrain epithelial cell proliferation. Among the growth factors, there has been particular interest in EGF and IGF-1. Studies using mouse models, including transplantation of uteri and vaginae from EGF receptor knockout mice, indicate that this receptor is required for the maximal fibromuscular stroma growth but not the epithelial cell proliferative response to estrogen.
IGF-1 and IGF-2 both stimulate the proliferation of human endometrial stromal cells via the type 1 IGF receptor. IGF-1 expression is greatest in the late proliferative and early secretory phase, whereas IGF-2 is most abundant in the midsecretory endometrium and decidua of the first trimester of pregnancy. The IGFs are bound by a family of binding proteins (IGFBPs) that modulate IGF activities in target tissues. One of the binding proteins, IGFBP-1, is a major secretory product of decidualized stromal cells, and it has been hypothesized to play a role in controlling trophoblast invasion.
Estrogen is the primary regulator of IGF-1 expression in the uterus, which occurs predominantly in the stroma. Estrogen also increases expression of IGF-1 receptors, which are primarily found on epithelial cells. The mitogenic response of the mouse uterus to estrogen is absent in mice with targeted deletion of the IGF-1 gene. Moreover, mice overexpressing IGFBP-1, which results in reduced IGF-1 bioavailability, have a blunted DNA synthesis response to estrogen treatment. Collectively, these observations suggest that IGF-1 produced in the uterine stroma in response to estrogen acts on the epithelial cells to stimulate DNA synthesis. However, tissue grafting experiments showed that an IGF-1 knockout mouse uterus responds to estrogen when placed into a wild-type animal, whereas a wild-type uterus placed into an IGF-1 knockout mouse showed minimal growth—indicating that systemic IGF-1 is sufficient to support estrogen-driven uterine growth. These findings substantiate the importance of IGF-1 in the uterine growth response to estrogen. Although uterine growth can occur in the absence of a paracrine IGF-1 system, these studies do not preclude a role for locally generated IGF-1 as a redundant signaling mechanism or the role of other locally produced growth factors.
Progesterone is a vital steroid hormone that is involved in secretory preparation of the endometrium for implantation, decidualization, and suppression of myometrial contractility during pregnancy. It too has been demonstrated to exhibit elaborate paracrine activities in the endometrium. Progesterone possesses antiinflammatory characteristics and promotes immunotolerance during implantation and pregnancy. It is also associated directly, or indirectly, with most of the secretory proteins made by the endometrium that support embryo development and implantation.
Progesterone signaling in the endometrium affects each of the cellular compartments, including the epithelium, stroma, and resident bone marrow (BM)-derived immune cells. It has become increasingly clear that progesterone has both direct and important indirect roles in both stroma and epithelial function. A primary role of progesterone after ovulation is to downregulate the actions of estrogen, which are unnecessary for normal secretory endometrial development and may, in fact, inhibit implantation. During the early proliferative phase, when progesterone concentrations are low, cytoplasmic PR is shielded from degradation by heat shock proteins and by interactions with coactivator proteins (SRC1 and 2) and chaperone proteins such as FKBP4 and FKBP5. In mice with a SRC2 conditional knock (Ncoa2–/–) , the females are infertile, similar to FKBP4 null mice, with each displaying implantation and decidualization failure. In women, both FKBP4 and FKPB5 expression normally increases in the secretory phase, possibly regulated by HOXA10. Interestingly, blunted increases in FKBP4 are seen in endometrium from women with infertility and endometriosis, regulated in part by changes in microRNA that regulate degradation of mRNA coding for FKBP4 and other progesterone-regulated genes. This mechanism may be involved in the phenomenon of progesterone resistance.
Much of our understanding of implantation and the paracrine actions of progesterone is based on studies in the mouse uterus. PRs are regulated by estrogen but also require the transcription factor Gata2. The action of progesterone begins with epithelial PR binding to progesterone followed by the induction of a key epithelial target gene, Indian hedgehog (IHH) . IHH is secreted and communicates with the endometrial stroma in a paracrine fashion, binding to the stromal receptor Patched-1 (Ptch1) , resulting in increased stromal transcription factor, chicken ovalbumin upstream promoter transcription factor II (COUP-TFII), with downstream effectors including bone morphogenic protein-2 (BMP2) and WNT4. Similar to mice lacking the PR chaperone protein FKBP4, COUP-TFII-deficient mice are infertile and exhibit excessive estrogen activity and failure of ERα downregulation. This pathway concludes with the induction of stromal HAND2 that is antiproliferative, blocking stromal fibroblast growth factor (FGF), which is required for the expression of epithelial estrogen receptors ( Fig. 9.5 ).
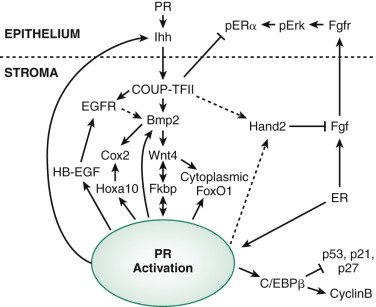
As mentioned earlier in this chapter, WNT4 is required for müllerian development and in adults is required for decidualization. WNT4 is also thought to be responsible for shuttling Forkhead box protein 1 (FOXO1), a transcription factor essential for decidualization, from the nucleus to the cytoplasm, preventing its apoptotic actions. Inadequate decidualization has been shown to be associated with implantation failure and associated with many critical genes.
Many of the stromal genes expressed in response to progesterone action including HOXA10 , heparin-binding EGF-like growth factor (Hbegf) , cyclooxygenase 2 (Cox-2, encoded by Ptgs2 ), and mitogen inducible gene 6 (Mig-6) , are induced through the IHH pathway. Mig-6 is a negative regulator of EGF receptor, and transgenic knockout mice for this protein have endometrial hyperplasia, suggesting this progesterone-regulated gene is an important brake for cell proliferation in the endometrium. The HOX gene, HOXA10, is essential for uterine development, but in the adult has been shown to regulate FKPB4 and is therefore also essential for progesterone action and implantation. HOXA10 also directly upregulates epithelial receptivity genes such as the ανβ3 integrin, which was shown to be required for attachment of the embryo in animal models. Transgenic mice lacking PR, HOXA10, Cox-2, AIRD1A, LIF, and other genes in transgenic mice have been found to exhibit decidualization defects and infertility.
The uterus is also a target for androgens. Androgen receptors (ARs) are expressed in the endometrium and myometrium, most prominently in the stromal cells during the proliferative phase and in the epithelial cells of the secretory phase. MAGE-11 was originally described as a primate-specific AR coactivator, reaches peak expression in the secretory phase, and colocalizes with AR. Interestingly, 5α-reductase types 1 and 2, which convert testosterone to dihydrotestosterone, are expressed in epithelial cells throughout the cycle. Estradiol treatment increases endometrial stromal AR expression in the Rhesus monkey, and estradiol in combination with either testosterone or progesterone augments epithelial and myometrial AR levels. Consistent with these observations, AR expression is elevated in the endometrium of women with PCOS; this finding may explain, in part, the implantation defects and early pregnancy loss reported to be associated with this syndrome. Androgens and ARs may also have essential roles for reproductive tract function in females, and this topic is becoming an active area of research.
Menstrual Cycle
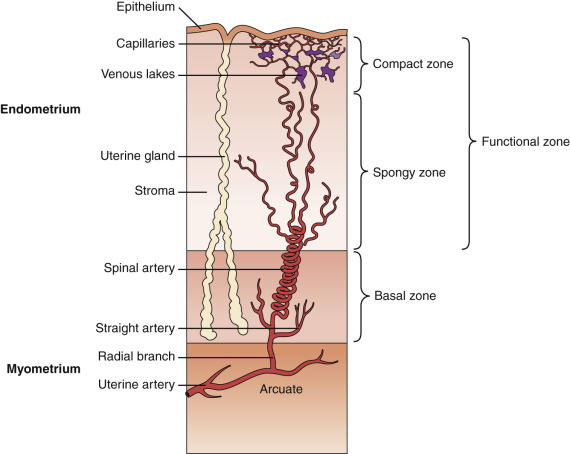
The endometrium undergoes progressive changes in response to preovulatory and postovulatory steroid hormones, to prepare for the possible implantation of the fertilized oocytes. In discussions of structure and function, the primate endometrium is commonly described as consisting of two major layers, the functionalis and basalis ( Fig. 9.6 ). The functionalis is a transient layer consisting of a compact zone that includes the stroma subjacent to the luminal epithelium and an intermediate spongy zone containing more densely packed tortuous glands, giving it a lacy histologic appearance. The basalis, or basal layer, lies beneath the spongy zone and adjacent to the myometrium. It contains the gland fundi and supporting vasculature, and can regenerate the functionalis endometrium after it is shed at menstruation. These endometrial layers are histologically definable during the secretory phase. However, in terms of function, the endometrium is best considered as a polarized gradient of cells with different phenotypes. The upper layers undergo a striking progression of histologic change during the menstrual cycle, whereas the basal region shows only modest alterations. Patterns of cell proliferation, programmed cell death, and gene expression also show gradients across the layers, as described in the next sections. The majority of epithelial cell proliferation occurs in the upper regions of the functionalis during the proliferative phase of the cycle. Proliferative activity in glands in the basalis is modest during the proliferative phase, with increased proliferative activity during the early to midsecretory phase, maintaining both ER and PR at a time when these receptors are normally depleted in the upper functionalis epithelium.
Histologic Changes
Early Proliferative Phase
During the early proliferative phase, the endometrium is usually less than 2 mm in thickness. Proliferation of cells in the basal zones and epithelial cells persisting in the lower uterine and cornual segments results in the restoration of the luminal epithelium by day 5 of the menstrual cycle. At that time, mitotic activity is evident in both the glandular epithelium and stroma. Remarkably, this recurrent “wound healing” process does not normally produce scarring. Endometrial stem cells capable of yielding progenitors of both the stromal and epithelial components of the endometrium presumably contribute to the regenerative process.
Rapid rejuvenation of the endometrium depends on many of the same factors that were involved in the ontogeny of the reproductive tract. WNT7A is expressed solely by the luminal epithelium and is a diffusible factor that triggers cell proliferation through complex pathways. Acting on the underlying stroma, soluble WNT7A binds to the receptor Frizzled (FZL) that phosphorylates the intracytoplasmic protein Dishevelled (DSH; Fig. 9.7A ). This protein inactivates glycogen synthase kinase β (GSKβ), turning off the breakdown of β-catenin by ubiquitination. Accumulation of β-catenin signals cell proliferation activities associated with endometrial growth acting as a transcription factor in the nucleus. The diffusion gradient of WNT7A downward into the growing endometrium is an attractive model for self-regulatory mechanisms to determine the growth of the endometrium.
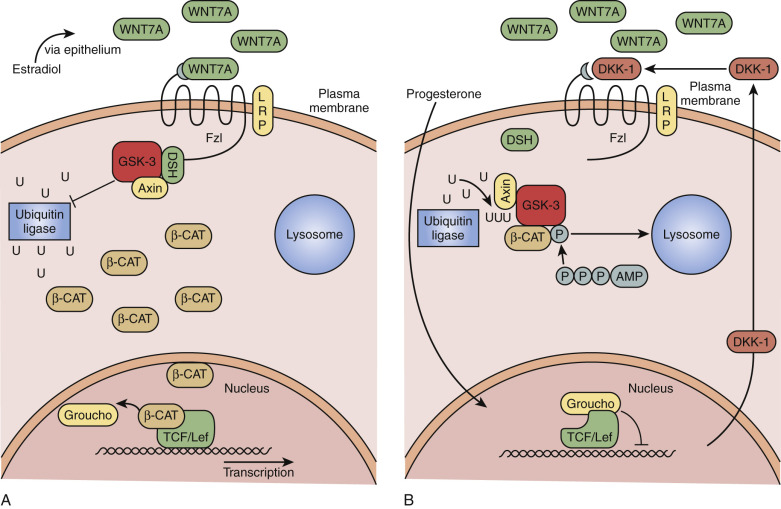
Counterregulatory mechanisms to disable WNT7A/FZL/DSH pathways include the action of progesterone, which stimulates secretion of a protein called Dickkopf-1 (DKK-1; see Fig. 9.7B ). DKK-1 binds to a coreceptor, LRP6, blocking the FZL receptor, turning off the action of WNT7A by preventing β-catenin accumulation. Defects in DKK-1 production have been described in endometriosis and reflect progesterone resistance and might explain the proliferative phenotype of the endometrium in this condition.
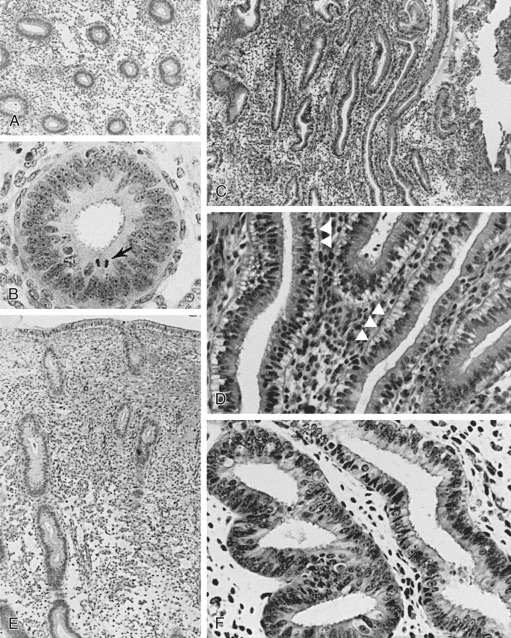
The glands during the early proliferative phase are narrow, straight, and tubular, lined with low columnar cells that have round nuclei located near the cell base ( Fig. 9.8 ). At the ultrastructural level, the epithelial cell cytoplasm contains numerous polyribosomes, but the endoplasmic reticulum and Golgi complexes of these cells are not well developed.
Late Proliferative Phase
The endometrium thickens in the late proliferative phase as a result of glandular hyperplasia and an increase in stromal extracellular matrix. The glands are widely separated near the endometrial surface and more crowded and tortuous deeper into the endometrium. The glandular epithelial cells increase in height and become pseudostratified as the time of ovulation approaches (see Fig. 9.8D ).
The effect of steroid hormones on the proliferation and secretion within the endometrium is highly dependent on the zones (basalis vs. functionalis layers). Studies from the rhesus macaque endometrium using specific labeling techniques show proliferation during the “proliferative” phase is confined to the functionalis layer.
Early Secretory Phase
Ovulation marks the beginning of the secretory phase of the endometrial cycle, although it should be noted that the endometrial luminal and glandular epithelial cells also display secretory activity during the proliferative phase. Mitotic activity in epithelial and stromal cells is restricted to the first 3 days after ovulation and is rarely observed later in the cycle. The nuclei of glandular epithelial cells and stromal cells develop heterochromatin in the early secretory phase (see Fig. 9.8 ). The glandular epithelial cells begin to accumulate glycogen-rich vacuoles at their base, displacing the nuclei to the mid regions of the columnar cells. Evidence for modest secretory activity is seen in histologic preparations as a light eosinophilic collection in the gland lumina. Ultrastructural studies of endometrial epithelia reveal abundant endoplasmic reticulum, and unusually large mitochondria with prominent cristae. A reticular network of argyrophilic fibers containing fibrillar collagens (collagen fiber type I and type III) is established in the stroma by the early secretory phase. Stromal edema contributes to the thickening of the endometrium at this time.
Midsecretory Phase
A characteristic feature of this phase of the cycle is the development of the spiral arteries. These vessels become increasingly coiled, as they lengthen more rapidly than the endometrium thickens. The endometrial glands are tortuous in the midsecretory and late secretory phases. Their secretory activity reaches a maximum about 6 days after ovulation, as reflected by loss of vacuoles from the epithelial cell cytoplasm (see Fig. 9.8E and F ).
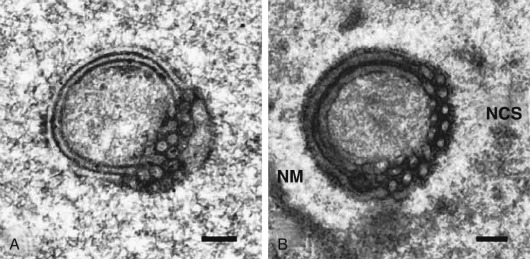
An ordered spherical stack of interdigitating tubules, the nucleolar channel system, appears transiently in the nucleoli of approximately 5% to 10% of the secretory phase epithelial cells between day 16 and 24 ( Fig. 9.9 ). The nuclear channel system is thought to form from an invagination of the inner nuclear membrane, providing a direct connection to the perinuclear space for transport of mRNA to the cytoplasm. Nopp140, a highly phosphorylated protein that associates with small nucleolar ribonucleoprotein particles that are required for RNA processing, appears to induce formation of this intranuclear endoplasmic reticulum. The nucleolar channel system forms in response to progesterone and is an ultrastructural hallmark of the secretory phase during the expected time of implantation.
Stromal cells around blood vessels enlarge and acquire an eosinophilic cytoplasm and a pericellular extracellular matrix in the mid- to late secretory phase. These changes, referred to as predecidualization to distinguish them from the further transformation of the stroma that occurs in a fertile cycle, subsequently spreads, accentuating the demarcation between the subepithelial compact zone and the spongy zone. Unlike many laboratory animal species, an embryonic signal is not required for initiation of decidualization in the human uterus.
The fact that the predecidual changes occur first near blood vessels suggests that humoral or local factors provoke them. Among the local factors may be interactions with decidual granular lymphocytes, also referred to as uterine natural killer (NK) cells. NK cells encircle arterioles and closely associate with stromal cells through contacts that are remarkably similar to gap junctions. At the ultrastructural level, the predecidual stromal cells display well-developed Golgi complexes and parallel lamellae of endoplasmic reticulum. Their surrounding matrix consists of laminin, fibronectin, heparan sulfate, and type IV collagen. The profile of gene expression decidualizing human stroma cells in vitro has been mapped by microarray analysis and includes induction of 121 genes, downregulation of 110 genes, and 50 genes showing a biphasic response.
The stromal cells of the midsecretory and late secretory phase also express a repertoire of proteins that promote hemostasis—including tissue factor (TF), a membrane-associated protein that initiates coagulation when it contacts blood, and plasminogen activator inhibitor type 1 (PAI-1), also known as Serpin E1, which restrains fibrinolysis. The pattern of gene expression prevents focal hemorrhage that might result from trophoblast invasion during implantation.
Premenstrual and Menstrual Phase
The main histologic features of the premenstrual phase are degradation of the stromal reticular network, which is catalyzed by MMPs; infiltration of the stroma by polymorphonuclear and mononuclear leukocytes; and “secretory exhaustion” of the endometrial glands, whose epithelial cells now display basal nuclei. Morphologic changes in the nuclei of granular lymphocytes, including pyknosis and karyorrhexis suggesting apoptosis, have been proposed to be some of the first events presaging menses; these changes occur prior to the breakdown of extracellular matrix and leukocyte infiltration. In the glandular epithelium, the nucleolar channel system and giant mitochondria characteristic of the early and midsecretory phases have vanished. The endometrium shrinks preceding menstruation, partly as a result of diminished secretory activity and the catabolism of extracellular matrix. A broader discussion of menstruation in the context of abnormal uterine bleeding (AUB) is discussed later in this chapter.
Menstruation
Menstruation, caused primarily by progesterone withdrawal, marks a failure to achieve pregnancy and the need to shed the specialized uterine lining that results from spontaneous decidualization. The uniqueness of this process is highlighted by the fact that, although circulating progesterone and estrogen levels decline with corpus luteum regression in nonfertile cycles in all mammals, menstruation appears almost exclusively in humans and some Old World primates. In menstruating species, moreover, tissues that respond to estrogen and progesterone such as the fallopian tubes, vagina, and breast do not shed as ovarian steroid levels decline. The molecular mechanisms triggered by progesterone withdrawal include activation of the NF-Kβ transcriptional pathway (a major target of cytokines) and the resulting expression of genes like endometrial bleeding–associated factor (EBAF), an anti-TGF-β cytokine that interferes with the action of other members of the TGF-β family that promote endometrial integrity. This orchestrated blockade of the actions of TGF-β appears to initiate many of the subsequent events of menstruation, including the elaboration of MMPs.
The functional zone of the human endometrium is supplied by spiral arterioles that, in contradistinction to the radial and basal arteries that feed them, are highly sensitive to steroid hormones. The classic studies of Markee that observed the structure of autologous endometrial transplants into the anterior eye chamber suggested that an ischemic phase caused by vasoconstriction of the arterioles and spiral arteries precedes the onset of menstrual bleeding by 4 to 24 hours. It has been proposed that bleeding occurs after the arterioles and arteries relax, leading to hypoxia or reperfusion injury.
The findings of Markee form the basis of the vasoconstriction model of menstruation. However, the notion that menstruation is a consequence of generalized vasoconstriction and hypoxia/anoxia in the functionalis is not supported by studies of endometrial perfusion that have failed to reveal any significant reduction in endometrial blood flow in the perimenstrual phase and during menstruation. In addition, an analysis of expression and localization of hypoxia-inducible factor (HIF), a heterodimeric transcription factor induced by hypoxia and thus a biochemical marker of reduced oxygen availability, was unrevealing. No upregulation of the two HIF component subunits, HIF-lα and HIF-lβ, was observed, and no nuclear localization of HIF subunits took place in the perimenstrual human endometrium. These studies do not, however, exclude the possibility of localized regions of vasoconstriction and hypoxia.
An alternative hypothesis to the vasoconstriction model is that menstruation is an inflammatory response engendered by the withdrawal of progesterone. The inflammation hypothesis is supported by two features: the prominent accumulation of leukocytes in the endometrium in the premenstrual phase, and the release of matrix-degrading enzymes characteristic of an inflammatory response. The hypothesis of progesterone withdrawal–induced inflammation is supported by the uterine inflammation observed in mice lacking PRs. Apoptotic cell death, which can be triggered by inflammatory mediators, occurs in the late secretory phase first in stromal cells and then gradually spreads throughout the functionalis. Rescue from apoptosis has been shown to occur in vivo with the administration of progesterone or exogenous human chorionic gonadotropin (hCG).
Changes in proteins involved in apoptosis appear to contribute to the regional programmed death in the endometrium. The antiapoptotic protein, Bcl-2, is prominently expressed in the glandular epithelium during the proliferative phase; expression declines in the secretory phase to reach low levels in the late secretory phase when apoptosis occurs. Studies report an inverse pattern of expression of survivin, a recently discovered inhibitor of apoptosis. Survivin binds to and blocks the effector cell death proteases, caspase-3 and -7. The activities of caspase-3, -8, and -9 are higher in the secretory phase. Low rates of survivin expression in glandular epithelium are found in the proliferative phase, rising to peak expression in the late secretory phase. The protein was localized to the nuclei of cells in the functionalis and the cytoplasm in cells in the basalis. This differential distribution may indicate that survivin is not capable of suppressing apoptotic death in the functionalis in the late secretory phase but performs this role in the basalis, consistent with the observed patterns of apoptotic cell death. Elevated levels of survivin in endometriotic lesions correlates with reduced apoptotic death of cells in these lesions.
Although the vasoconstriction and inflammation hypotheses of menstruation might appear to be distinct, there are several overlapping biochemical features of hypoxia and inflammation, including the release of proinflammatory cytokines and apoptotic cell death that tend to blur the distinctions between these models. The vascular changes in the endometrium in the perimenstrual phase, resulting either from ischemia/hypoxia or from inflammatory reaction, lead to extravasation of blood. Autophagy and heterophagy are evident, as is apoptotic cell death. The superficial endometrial layers become distended by the formation of hematomas; fissures subsequently develop, leading to the detachment of tissue fragments and the ultimate shedding of the functionalis. The resulting menstrual effluent contains the fragments of tissue mixed with blood, liquefied by the fibrinolytic activity of the endometrium. Clots of varying size may be present if blood flow is excessive.
The inflammatory components of menstruation may be essential for the rapid restoration of tissue integrity that occurs following endometrial sloughing. The withdrawal of progesterone, as a well-known antiinflammatory mediator of the secretory phase, likely participates in the onset of inflammatory changes, including induction of MMPs, urokinase-type plasminogen activator and tissue-type plasminogen activator (uPA and tPA), and PAI-1 expression. At menstruation and with progesterone withdrawal, cyclooxygenase-2 (COX-2) is dramatically elevated via NFkB, with induction of prostaglandins and lipoxygenases (LOX). One LOX, LOX15, is expressed with progesterone withdrawal. Since LOX15 is responsible for production of the antiinflammatory eicosanoid lipoxin A4 (LXPA4), expression may help curtail inflammatory responses. Surprisingly, LXPA4 has also shown to be a potent estrogen receptor agonist and could function to facilitate repair of the endometrium, particularly during menstruation, when circulating estrogen levels are low.
The duration of menses in ovulatory cycles is variable, generally 4 to 6 days and usually similar from cycle to cycle in any individual ovulatory woman. The duration of flow is considered to be abnormal if it is less than 2 days or more than 7. The amount of blood lost in a normal menses ranges from 25 to 60 mL, being greater when coagulation and platelet disorders are present. Loss of more than 60 mL per month is associated with iron deficiency anemia.
Menstruation and the subsequent regeneration of the functionalis layer are notable for the lack of development of synechiae. The majority of cases of uterine synechiae causing Asherman syndrome occur following pregnancy-related curettage, especially when curettage is performed during the first 4 weeks of the postpartum period when the uterus is particularly vulnerable.
Stem Cells and Telomerase in Endometrial Renewal
Menstruation and cyclic repair of the endometrial lining requires both stem cells and an adaptation involving telomerase that imbues the endometrium with near immortality, compared with other tissues in the body. In 2004 the endometrium of women who had undergone BM transplantation was examined and found to contain endometrial epithelial and stroma cells that had clearly been derived from the donor’s BM. This experiment demonstrated that BM-derived stem cells can traffic to and engraft in the endometrium, and secondly, that these undifferentiated BM stem cells undergo both mesenchymal and epithelial transformation adopting a phenotype indistinguishable from their host organ. This original observation was later confirmed in the mouse, using male BM donors and female recipients, proving that these were not endometrial stem cells that reside in the BM, but rather BM-derived pluripotent stem cells.
Endometrial derived pluripotent cells, whether native to the endometrium or derived from the BM, appear to have unusual properties that may revolutionize how we think about stem cell research. These cells have been manipulated in the mouse model to differentiate into neuronal cell types with the potential to produce dopamine, and pancreatic β cells that produce insulin. Mesenchymal stem-like cells that express CD140b and CD146 have also been identified by Gargett et al. and can be differentiated into adipocytes, osteocytes, chondrocytes, myocytes, and endothelium. Based on these early reports, the ready access of stem cells of endometrial origin could supplant the necessity to perform BM biopsy and offers the promise of a self-renewing source of autologous stem cells for grafting of a wide variety of cell types that might cure such human diseases such as Parkinson or type I diabetes, as well as treatment for Asherman syndrome with the possibility of endometrial renewal.
Early methods for identifying endometrial stem cells began with the study of retention of cells labeled with 5-bromo-2-deoxyuridine (BrdU). These rare, highly proliferative and undifferentiated quiescent populations of cells, as pointed out by Gargett, maintain properties of clonicity, colony forming unit activity, and ability to reconstitute their tissue of origin. Hoechst 33342 exclusion to identify the side population (SP) cells have been used to show that in the endometrium, these SP cells are maintained at a constant level (~1%) throughout a woman’s reproductive life. Interestingly, it was recently suggested that BM-derived cells do not contribute to this population of SP cells in the endometrium, suggesting they are a resident to the endometrium and therefore provide an endogenous source of self-renewing stem cells to the endometrium.
Specific cell surface markers have been identified in endometrial mesenchymal stem cells (MSGs). These CD146 + PDGFR-β − cells only represent less than 2% of endometrial stromal cells and are found in a perivascular location, similar to other BM–derived cells. These cells have increased proliferation capacity and may be important in reconstitution of the endometrium following menstruation.
The endometrium, unlike other tissues in the body, does not appear to age. Despite up to 450 cycles of menstruation and regeneration, the endometrium of the typical woman continues to renew itself with remarkable reliability. Age is related to telomere length, which in other tissues determines the life-span of a cell. Endometrium expresses the enzyme telomerase, at levels similar to certain cancers. Levels are cycle dependent, with higher levels in the proliferative phase reaching their nadir during the midsecretory phase. Interestingly, telomerase is found in the epithelial but not the stromal compartment. Epithelial stem cells are thought to participate in endometrial renewal following menses, arising from the basalis layer. These cells appear to express stage-specific embryonic antigen 1 (SSEA-1, or CD15), a Lewis X epitope found on embryonic stem cells. The endometrial cells expressing this epitope had greater telomerase activity than nonexpressing cells.
Based on recent reports, telomerase may be indirectly regulated by estrogen, through Wnt pathways involving β-catenin expression. In women and baboons with endometriosis and progesterone resistance, telomerase levels appear to be increased, suggesting a possible link to this disease and the pathophysiology of its chronicity and tendency to recur.
Vascular Remodeling and Angiogenesis
Angiogenesis , the formation of new blood vessels from preexisting vessels, rarely occurs without injury or disease in the normal adult, except in the female reproductive tract and ovary. Here the cyclic processes of endometrial shedding and regeneration and corpus luteum formation entail remarkable changes in vessel growth and remodeling. The angiogenic process involves multiple steps and is tightly regulated by activators and inhibitors. There are four phases of the endometrial cycle when important events relating to angiogenesis occur: (1) at menstruation, when there is repair of ruptured blood vessels; (2) during the proliferative phase, when there is rapid growth of endometrial tissue; (3) during the secretory phase, with the development of the spiral arterioles that feed a subepithelial capillary plexus; and (4) in the premenstrual phase, when there is evidence for vascular regression. If this angiogenic remodeling program is not properly executed, abnormalities in endometrial function can result, including menorrhagia.
Angiogenesis during the proliferative phase is by vessel elongation. In the secretory phase, intussusception appears to account for the increase in vessel branching; this proliferation of endothelial cells inside of vessels ultimately produces a wide lumen that can be divided by transcapillary pillars, or alternatively lead to capillary fusion or splitting. Although most prominent in the late menstrual and early and late proliferative phase, endothelial cell proliferation is continuous during the menstrual cycle. Thus vessel growth continues during the secretory phase, despite the fact that the surrounding endometrial tissue has ceased to grow, resulting in the coiling of spiral arterioles.
Endometrial angiogenesis and vessel remodeling are directed by a network of signaling molecules and receptors that include members of the vascular endothelial growth factor family, FGFs, angiopoietins, angiogenin, and the ephrins and their cognate receptors—which can also exist as secreted ligand-binding domains that function as inhibitors as a result of alternative splicing. Although the temporal and spatial patterns of expression of several other angiogenic factors and their receptors have been defined in the endometrium, the specific roles of each of these factors in the endometrial angiogenesis–vessel remodeling cycle remain to be elucidated.
Of the members of the vascular endothelial growth factor family that includes VEGF-A, VEGF-B, VEGF-C, and VEGF-D, VEGF-A is most important for endometrial angiogenesis. VEGF-A acts on two different receptors: VEGF receptor-2 (VEGFR2), which may play the dominant role in signaling endothelial cell proliferation; and another tyrosine kinase receptor, VEGFR1 (also known as FLT-1), which may play the dominant role in mediating VEGF effects on vascular permeability. Both receptors are present on endothelial cells. VEGFR2, also known as kinase domain receptor (KDR), has been detected in stromal and epithelial cells of the premenstrual endometrium; this presence suggests actions on nonvascular compartments.
The premenstrual phase is characterized by a dramatic upregulation of VEGFR2 receptor in stromal cells of the superficial layers of the endometrium in response to progesterone withdrawal. The action of VEGF on VEGFR2 may participate in the increased expression of MMP-1 in the stromal in the premenstrual phase.
VEGF-A expression is detectable in glandular epithelial and stromal cells in the proliferative phase, stimulated by estrogen through the actions of hypoxia induced factor-1α (HIF-1α). HIF-1α is also induced by prostaglandins E2, which is maximally produced at the time of menstruation. VEGF released from neutrophils in intimate contact with the endothelial cells is thought to stimulate endometrial vessel growth. It is also present in uterine NK cells. During the secretory phase, VEGF-A can be identified in surface epithelial cells, which presumably secrete it into the uterine cavity. The release of VEGF from subepithelial NK cells has been suggested to play a role in directing the development of the subepithelial capillary network in the secretory phase.
VEGF-A has four common isoforms. It has four common splice variants (VEGF121, VEGF165, VEGF189, and VEGF 206). After ovulation, there is a remarkable shift in VEGF-A isoforms expressed in the uterus with the appearance of VEGF-A189 in the perivascular stromal cells; the VEGF-A189 isoform can be processed by proteolytic cleavage by plasminogen activator. VEGF-A189 increases vascular permeability acting on VEGFR1, while its processed form binds to the VEGFR2 receptor, which mediates the mitogenic action on endothelial cells.
The highest VEGF-A levels are found in the menstrual phase, probably a response to proinflammatory cytokines. The surge might also be attributed to focal hypoxia, which is a potent stimulus to VEGF-A gene transcription. Expression of VEGFR1 and VEGFR2 is also greatest in the menstrual phase. The elevated levels of VEGF and cognate receptor expression at this time are presumed to be important for vessel repair and the preparation for angiogenesis in the proliferative phase. Notably, the functional activity of VEGFR2 (as assessed by receptor phosphorylation), a signature indicating ligand activation, is relatively low in the early menstrual phase when levels of soluble VEGFR1 (sFLT-1), which sequesters VEGF, are highest. VEGFR2 receptor phosphorylation increases substantially in the late menstrual phase, remaining modestly elevated in the early and late proliferative phase when sFLT-1 levels decline.
The FGF family of proteins may also participate in endometrial angiogenesis through interactions with the VEGF system. FGFs upregulate VEGFR2 and VEGF-A expression, and in a feed-forward loop, VEGF-A promotes the release of FGFs from the extracellular matrix. Basic FGF is a potent stimulus for ανβ3 integrin expression. This integrin is present at the site of active angiogenesis and fundamental to endothelial invasion and vessel elongation during angiogenesis.
The angiopoietins (Ang) regulate vessel stability. Ang-1, expressed in vascular smooth muscle cells, binds to a cognate receptor, Tie-2, on endothelial cells, resulting in vessel stabilization. Ang-2 is a physiologic antagonist of Ang-1. It also binds to the same Tie-2 receptor. Vessels atrophy when Ang-2 acts in the absence of VEGF-A, whereas angiogenesis is promoted in the presence of VEGF-A. In situ hybridization studies indicate that Ang-1 expression is most abundant in the glands and stroma of the early and midproliferative phase and reduced in the late proliferative phase. Ang-2 expression is detected in the glands and stroma throughout the cycle, with highest expression in the early proliferative and mid- to late secretory phases. In endometrium from women with menorrhagia, Ang-1 expression is consistently downregulated; as a result, the ratio of Ang-1 to Ang-2 is reduced, which contributes to vessel instability.
Angiogenin is a heparin-binding molecule that is expressed by endometrial epithelial and stromal cells at greatest levels in the mid- to late secretory phases and the decidua of early pregnancy. Angiogenin is thought to contribute to the proliferation of vascular smooth muscle cells around the spiral arterioles. Like VEGF-A, expression of angiogenin is stimulated by hypoxia. It is also increased by progesterone.
Ephrins , a family of molecules and their cognate tyrosine kinase receptors, are believed to guide endothelial cells to specific targets. Ephrins have been detected in endometrial endothelial and stromal cells, but the functional roles of these molecules and their receptors in the uterus remain to be clarified.
The physiologic consequences of angiogenesis are reflected in changes in endometrial blood flow. By measuring the clearance of radioactive xenon gas, highest endometrial perfusion was reported between days 10 and 12 and days 21 and 26 of the cycle. Microvascular perfusion has been assessed by laser Doppler fluximetry with transvaginal placement of a fiberoptic probe into the uterine cavity. With use of this technique, endometrial perfusion was found to be highest during the proliferative phase and the early secretory phase, not too dissimilar from the finding based on xenon clearance. Uterine blood flow is greatest in the fundus, and higher flow rates are associated with better outcomes in assisted reproduction. Notably, diminished uterine blood flow has not been found in the perimenstrual period, but these methods cannot easily identify localized areas of vasoconstriction.
Extracellular Matrix Remodeling
The biochemical basis for the dramatic structural changes in the endometrium in the perimenstrual period includes the action of specific matrix-degrading proteases, the MMPs. Studies on human endometrial explants in culture demonstrated that degradation of the extracellular matrix occurs in the absence of progesterone and estrogen, which suppress expression MMPs. Moreover, this degradative process can be blocked by MMP inhibitors, but not by inhibitors of lysosomal cysteine proteinases—directly implicating MMPs in the catabolism of the endometrial extracellular matrix.
Enzymes of the fibrinolytic system, urokinase and tissue plasminogen activator, are increased in the endometrium as progesterone is withdrawn in the perimenstrual period. Moreover, PAI-l expression is reduced, allowing the plasminogen activators to activate plasmin and proteolytically cleave and activate the latent MMP proenzymes.
MMPs represent a large family of proteinases that play a major role in remodeling of the extracellular matrix ( Fig. 9.10 ). In situ hybridization and immunocytochemistry have been used to map the expression of MMPs and the endogenous inhibitors, tissue inhibitors of the matrix metalloproteinases (TIMPs), in the primate endometrium. Cell-specific and menstrual cycle–specific patterns were revealed, with the most profound changes occurring during the perimenstrual period. After ovulation, the expression of interstitial collagenase (MMP-1), stromelysin-1 (MMP-3), and stromelysin-2 (MMP-10) in the endometrial stroma is essentially restricted to the perimenstrual and menstrual phase.
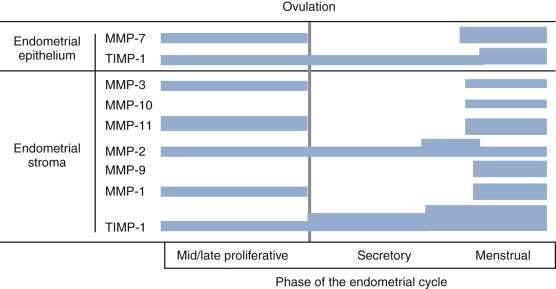
Other MMPs are detected during the proliferative and secretory phases but are significantly increased in expression perimenstrually. These include the type IV collagen-degrading enzymes, MMP-2 and MMP-9. The membrane-bound MMP, MMP-14 (which activates MMP-2), is detected during menstruation in stromal inflammatory cells and epithelial cells. TIMP-1, which is detectable in the endometrium throughout the cycle, is increased in the stroma, epithelium, and arterioles at menstruation.
The importance of progesterone withdrawal in regulating endometrial MMPs and the different temporal patterns of expression have been well-documented in in vivo and in vitro systems. In a primate model in which hormone levels were manipulated by steroid implants, progesterone withdrawal resulted in upregulation of MMP-1, -2, -3, -7, -10, -11, and -14. It is important to note that the expression of MMPs in the endometrium is heterogeneous.
At the start of menstruation, MMP-1 is found in patches of stromal cells in the superficial zone; these patches are colocalized with areas of reduced stromal and epithelial expression of estrogen and PR and focal disruption of the extracellular argyrophilic fibrillar network—reflecting the degradative activity of MMP-1. As the process of menstruation proceeds, MMP-1 expression spreads to include the entire functionalis. MMP-2 and MMP-3 expression is also limited to the stromal cells in the functionalis. During menses, MMP-1, -2, -3, and -9 localize primarily in and around arteriolar walls. The heterogeneity of MMP expression suggests that MMP gene transcription is under the control of local rather than systemic (steroidal) factors. In other words, steroids are indirectly influencing MMP expression.
Progesterone, particularly in the presence of estradiol, can suppress expression of certain MMPs (i.e., MMP-1, -2, -7, -9, and -11) in endometrial explant culture. This action is most likely explained by changes in autocrine/paracrine signals—particularly proinflammatory cytokines or members of the transforming growth factor family, which respectively are potent inducers and suppressors of MMP gene transcription. In culture systems, IL-lα has been implicated as the mediator of MMP-1, MMP-3, and MMP-7 expression in response to withdrawal of progesterone. Neutralizing antibodies to TGF-β prevent the action of progesterone in blocking MMP-3 and MMP-7 expression.
EBAF is the orthologue of the murine gene named Lefty and another likely candidate for a progesterone-regulated cytokine controlling MMP expression. Lefty was originally identified in human endometrium as a gene upregulated in the late secretory and menstrual phases of the normal cycle, being absent in the proliferative, early, and midsecretory endometrium. EBAF expression, which is predominantly found in the endometrial stroma and to a much lesser extent in the glandular epithelium, is suppressed by progesterone. Interestingly, endometrium from women with a history of abnormal bleeding and endometriosis expressed Lefty at unusual times including the proliferative, early, and midsecretory phases.
Unlike other members of the TGF-β family that promote the formation and stability of the extracellular matrix, EBAF downregulates the elaboration of collagen in association with reduced expression of connective tissue growth factor, while upregulating expression of collagenolytic and elastinolytic enzymes. These actions of EBAF are the result of antagonism of the SMAD signaling pathway that is activated by the other TGF-β growth factors. Thus the decline in progesterone and estradiol in the late luteal phase initiates alterations in the endometrium that include upregulation of proinflammatory cytokines (some of which may be contributed by immune cells that accumulate in the endometrium) and a natural TGF-β antagonist. The collective result is focal and then widespread expression of matrix degrading enzymes that result in the remodeling of stroma and blood vessels in the functionalis.
Lysosomal involvement in the process of menstruation has been proposed because of three observations: an increase in the abundance of lysosomes in the endometrium during the late secretory phase, the cytochemical demonstration of acid phosphatase in the perimenstrual endometrium, and the high specific activity of certain lysosomal hydrolases in endometrial tissue in the menstrual phase. However, inhibitors of these enzymes, leupeptin and E-64, do not prevent the progesterone withdrawal–induced breakdown of extracellular matrix in endometrial explants as do the inhibitors of MMP activity. These observations suggest that lysosomal proteinases are not major contributors to the remodeling of the perimenstrual endometrium.
Vasoactive Substances
The endothelins are a family of potent vasoconstrictors produced by endothelial cells that act on two types of receptors present on vascular smooth muscle. Endothelin-1, produced by endometrial epithelial or stromal cells, may act on spiral artery smooth muscle cells to promote vasoconstriction. Enkephalinase, a membrane-bound metalloendopeptidase, degrades endothelin-1 and other vasoactive peptides, and is present in highest levels in the midsecretory endometrium. Expression of the gene encoding enkephalinase is upregulated by progesterone. The decline in progesterone levels at the end of the luteal phase results in a subsequent fall in enkephalinase, which prolongs the biological life of endothelin-1. Vasopressin may also function as a vasoconstrictor in the endometrium during the menstrual phase of the cycle.
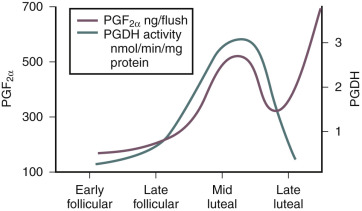
The production of prostaglandins, particularly PGF 2 α and other eicosanoids in the endometrium, is enhanced by lysosomal phospholipases that liberate the arachidonic acid that accumulates in endometrium during the secretory phase; in turn, arachidonic acid is metabolized into prostanoids (see Chapter 4 ). The premenstrual decrease in progesterone is also followed by an induction of the prostaglandin synthase, COX-2, and decline in 15-hydroxyprostaglandin dehydrogenase activity, which inactivates PGF 2 α. This induction of the prostaglandin synthase, COX-2, and the decline in 15-hydroxyprostaglandin dehydrogenase leads to increased production and bioavailability of PGF 2 α, which triggers myometrial contractions that compress the endometrial vasculature and promote hemostasis ( Fig. 9.11 ). Increased PGE2 coupled with hypoxia stimulates IL8 expression with a role in endometrial repair.
Hemostatic and Fibrinolytic Mechanisms
The relative activities of the hemostatic and fibrinolytic systems in the endometrium are shifted in the perimenstrual period such that clotting activity is reduced and fibrinolytic activity is increased. Consequently, menstrual fluid does not normally clot, even on prolonged storage. Decidualized stromal cells express TF, the primary trigger of thrombin formation and hemostasis, under the influence of progesterone. Expression of TF is expressed in the endometrial stromal cells along with another hemostatic factor, PAI-1, under the influence of progestins, which suppresses MMP production. TF production by the decidualized stromal cells declines with withdrawal of progesterone, along with the increase in MMP production.
The endometrial fibrinolytic system includes uPA and tPA, which cleave plasminogen to yield the fibrinolytic enzyme, plasmin. Progesterone reduces expression of urokinase and increases that of PAI-1 in cultured endometrial cells. Removal of progesterone or the addition of the antiprogestin, RU486, reverses these responses.
Endometrium in the Cycle of Conception and Pregnancy
Secreted Proteins of the Endometrium
In preparation for pregnancy, the endometrium produces a large number of secreted proteins that are collectively referred to as the endometrial secretome. These proteins serve critical autocrine, paracrine, and juxtacrine roles for the developing endometrium and embryo. The uterine glandular epithelium also secretes glycogen that is thought to serve as a histiotrophic nutrition source for the embryo prior to 10 weeks gestation. In addition to macromolecules, the endometrium also is likely to selectively secrete many small molecules, since it is well endowed with many members of the adenosine triphosphate–binding cassette transporter protein family, which are involved in the regulated secretion of a variety of small molecules, including drugs, lipids, and conjugated molecules.
The endometrial glandular secretions provide early nourishment for the embryo and signal the invading trophoblast. A vascular circulation to the embryo does not occur until the end of the first trimester, making these uterine secretions of critical importance. Endometrial secretory proteins can be detected in the lumen of the uterine cavity and some are found in the general circulation. A remarkable increase in secretory activity is associated with the luteal phase and early pregnancy, primarily in the glandular epithelial cells and later the decidua. These secreted proteins are either directly or indirectly regulated by progesterone, and most are implicated in regulation of embryo implantation, trophoblast invasion, and/or early embryo survival. Therefore an understanding of the mechanisms of progesterone action allows significant insight into endometrial function.
Glycogen
Glycodelin , the most abundant products of the secretory phase endometrial glands and decidua, is known by several names: progesterone-associated endometrial protein, α-uterine protein, pregnancy-associated endometrial α2 globulin, endometrial protein 15, chorionic α2 macroglobulin, and placental protein 14 (an erroneous designation because glycodelin is an endometrial/decidual protein). The mature form of glycodelin contains 162 amino acid residues and is 17.5% carbohydrate by weight. It has extensive structural homology with the β-lactoglobulins and, to a lesser extent, with retinol-binding proteins.
Glycodelin is a glycoprotein with four isoforms. Glycodelin A is the major progesterone-regulated protein secreted into the uterine lumen. However, its functions with respect to the endometrium, implantation, and pregnancy are still largely unknown. Glycodelin A is a potent inhibitor of sperm-egg binding, and researchers have postulated that it may play a role as an immunomodulator because of its ability to suppress NK cells. Glycodelin has been shown to limit trophoblast invasion in vitro by suppressing MMP production by cytotrophoblast. The absence of glycodelin A during the time of fertilization and its appearance during the time of implantation and placentation are consistent with these proposed functions.
Glycodelin levels in uterine flushings are tightly correlated with the histologic date of the endometrium ( Fig. 9.12 ). It is not detectable in significant amounts in uterine flushings during the follicular and early luteal phase. Six days after ovulation, however, the levels rise rapidly to reach a concentration 100 times higher than plasma levels. In peripheral serum, glycodelin appears 5 days after ovulation, reaching peak concentrations in nonfertile cycles at about the time of menstruation; levels reach a nadir during the midfollicular phase of the subsequent cycle. In a cycle of conception, glycodelin levels increase rapidly after implantation, reaching a maximum at 8 to 10 weeks of gestation and subsequently declining in a pattern that mimics the changes in hCG.
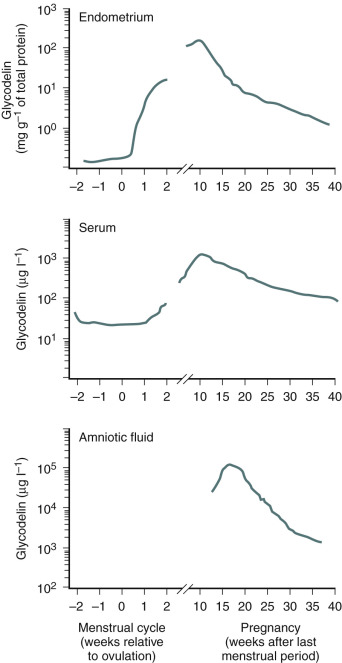
The discordant pattern of glycodelin and progesterone in serum may reflect the slower turnover of the protein. Levels of glycodelin in serum fail to rise in women using combination-type oral contraceptives and in some patients with luteal-phase defects. There is good—but not perfect—correlation between the progestational activity of steroids and their ability to stimulate endometrial glycodelin synthesis. Relaxin has also been implicated as a stimulus for glycodelin expression. Specific histone deacetylase inhibitors have been shown to potentiate the action of progesterone on both endometrial epithelium and stroma. The histone deacetylase inhibitor trichostatin A (TSA) has been shown to induce glycodelin in Ishikawa cells. Using an in vitro model of implantation, Uchida et al. showed that induction of glycodelin by another histone deacetylase inhibitor enhanced placental cell (JAR) spheroid attachment to Ishikawa cells using an in vitro model of implantation. This and other studies suggest a role for glycodelin in both the differentiation and function of the receptive endometrium.
Glycodelin has been shown to be decreased in the endometrium of women with infertility, including those with luteal phase defect and PCOS. In women exposed to the levonorgestrel containing intrauterine device (IUD) or the Yuzpe emergency contraception regimen, glycodelin expression was unaffected or increased.
Insulin-like Growth Factor Binding-protein I
IGFBP-1, also known as pregnancy-associated αl-protein and placental protein 12, is a major secretory product of decidual cells. It is one of several proteins that bind IGF-1 and IGF-2, affecting the ability of these growth factors to interact with the IGF receptors. Consequently, the binding proteins can have significant roles in modulating IGF effects. The protein undergoes posttranslational modification by phosphorylation, which increases its affinity for IGF-1 and therefore its ability to neutralize IGF-1 action.
IGFBP-1 derived from the decidua has been proposed to control the invasion and proliferation of trophoblast cells during implantation and placentation by sequestering IGFs. In a transgenic mouse model, overexpression of IGFBP-1 in the decidua resulted in abnormal placental morphology due to defects in trophoblast invasion and differentiation. Because IGFBP-1 contains the Arg-Gly-Asp (RGD) motif recognized by cell surface integrins that bind fibronectin, its actions may be more complex than simple IGF sequestration when it is presented to cells that express the RGD-binding integrins.
Stromal cell IGFBP-1 mRNA levels are regulated by progesterone as well as by hypoxia, IGFs, insulin, relaxin, and other growth factors. Both IGF and insulin decrease decidual IGFBP-1 release, while relaxin increases its release in a dose-dependent manner. HOXA10 may have a mild impact on IGFBP-1 expression, but in the presence of the forkhead/winged-helix transcription factor, FOX01, IGFBP-1 is markedly stimulated. FOX01, prolactin, and IGFBP1 are all induced during decidualization, and likely are involved in the progesterone-mediated stromal differentiation and prevention of apoptosis in preparation for nidation.
Intrauterine microdialysis studies revealed that IGFBP-1 is released into the uterine lumen in the late secretory phase (10 days after ovulation or later) and increased secretion in response to hCG. IGFBP-1 is increased between midsecretory to 6 weeks of pregnancy, as demonstrated by DNA microarray analysis on endometrium from ectopic pregnancy. IGFBP-1 levels then decline in the second trimester only to rise again in late pregnancy, also accumulating in amniotic fluid. Pregnancy termination with the antiprogestin RU486 causes a marked decline in IGFBP-1 levels before a decrease in hCG levels, confirming the progesterone dependence of IGFBP-1 production by decidual cells.
Defects in IGFBP-1 have been noted in certain conditions associated with infertility and pregnancy loss, including PCOS and endometriosis. Such observations may reflect an altered endocrine milieu, higher insulin levels, or a relative progesterone resistance in these conditions (discussed in subsequent text). In vitro, insulin inhibits the normal process of endometrial stromal differentiation (decidualization). In addition, hyperinsulinemia downregulates hepatic IGFBP-1, resulting in elevated free IGF-I in the circulation. Thus the elevated androgens and estrogen seen in PCOS, along with decreased progesterone in the absence of ovulation, likely contribute to endometrial dysfunction, infertility, increased miscarriage rate, endometrial hyperplasia, and endometrial cancer common in women with PCOS.
Osteopontin
Osteopontin (OPN) is a member of the SIBLING family of proteins. Each of the proteins in this large family contain the three amino acid sequence arg-gly-asp (RGD) and have binding sites specific for two major cell surface receptors, ανβ3 and CD44. OPN is a 70-kDa glycosylated phosphoprotein secreted by the glandular epithelium and is expressed during the midsecretory phase, localized to the luminal endometrial epithelium. OPN is regulated by progesterone. The secretion of OPN and subsequent binding to the luminal surface suggests direct interaction is occurring between integrins and this molecule, with a role as a “sandwich” ligand that serves as a bridge between surface receptors on the endometrial and embryonal surfaces. Alternative roles for OPN and these receptors include the prevention of complement fixation as part of a protective mechanism involving the innate immune system.
Prolactin
Prolactin is produced by both the endometrium and the myometrium. Serum levels of prolactin do not change across the menstrual cycle but rise markedly during the first trimester of pregnancy in parallel with the decidual response, reaching peak concentrations at 15 to 20 weeks of gestation. Prolactin, like IGFBP-1, is a downstream biomarker of decidualization of human endometrial stromal cells. Regulators of decidualization include ovarian steroids, cAMP or forskolin, IL-11. Recent studies suggest upstream mediators of decidualization may regulate prolactin expression, including various proteins expressed during the secretory phase, including ghrelin, IGFBP related protein-1, leptin, and oncostatin-M. The proliferation of endometrial cells is inhibited during decidualization, perhaps through stromal factors, such as IL-6 and oncostatin-M. Prolactin’s role in the decidualization process remains unclear. Prolactin receptor (PRL-R) is expressed on the endometrial stroma, and while PRL-R knockout mice had defects in decidualization, the phenotype including reduced expression of LIF, amphiregulin (AMP), HB-EGF, COX-1, COX-2, PPARδ, HOXA10, cyclin-D3, VEGF, and its receptors, Flk-1 and neuropilin-1, could all be rescued by exogenous progesterone. This suggests that ovarian PRL-R stimulation by prolactin inducing progesterone secretion is a critical event, rather than any direct effect of prolactin acting directly on the decidualizing stroma. A role for uterine prolactin controlling the NK cells has recently been suggested. Prolactin produced by the decidua during pregnancy accumulates in the amniotic fluid, where it has been postulated to have effects on osmoregulation and fetal lung development.
Endometrial Preparation for Implantation
The timing of conception is exquisitely synchronized to ovarian events. With the rise in estrogen associated with follicular development, the endometrium proliferates. After ovulation, rising progesterone from the nascent corpus luteum transforms this proliferative endometrium into a secretory structure and becomes receptive for the newly fertilized ovum ( Fig. 9.13A and B ; see Chapter 7 ). In the appropriate endocrine milieu, the growing embryo will interact with the surface epithelium and rapidly invade into the underlying stroma, initiating gestation ( Fig. 9.13C ). Implantation can be divided into distinct and separate stages, timed precisely to the steroid-mediated changes ongoing in the endometrium ( Fig. 9.14 ). Following ovulation, the ovum enters the fallopian tube where fertilization occurs. The early cell divisions ensue and the embryo enters the uterine cavity at the morula stage, approximately 3 to 4 days after being fertilized. Implantation begins about the sixth to seventh day after fertilization. The initial attachment reaction (apposition) may be the rate-limiting step, and failure to adhere may preclude the subsequent stages of implantation.
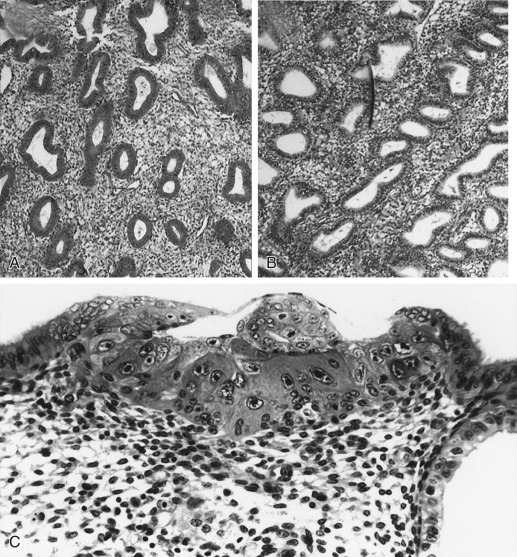
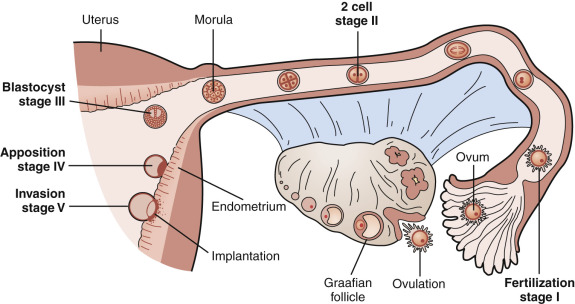
Studies in experimental and domestic animals have demonstrated the synchronous development of the embryo and endometrium that is required for normal implantation and development to occur. In laboratory animals, there is a discrete “window” of time for implantation, which in some species lasts for only a matter of hours. The concept of a “window” for implantation has also been demonstrated in the human female, with a molecular basis that continues to be elucidated.
Uterine receptivity is defined as the period of endometrial maturation during which the trophectoderm of the blastocyst can attach to the endometrial epithelial cells and subsequently proceed to invade the endometrial stroma and vasculature. The transition from the nonreceptive to the receptive state is determined by the expression of membrane-bound, soluble, and secretory factors that support trophoblast attachment and subsequent migration. Factors expressed during this temporal window have been exploited as biomarkers of the receptive state.
The timing of implantation has been examined with increasing scrutiny over the past 50 years. In the 1950s, luteal phase hysterectomy samples in pregnant subjects suggested that embryos did not attach until day 20 of a 28-day cycle. Using donor oocyte-derived embryos transferred into hormonally prepared recipients, Bergh and Navot later suggested that implantation occurred between cycle days 20 and 24. These and other studies of donor oocyte cycles also highlight the fact that replacement of estrogen and progesterone, alone, are sufficient to prepare the endometrium for embryo implantation, and that the length of progesterone exposure determines the window of endometrial receptivity.
More recently, by examining the time of pregnancy in 221 normal cycling women who were attempting pregnancy, Wilcox et al. showed implantation normally occurred between 7 and 10 days after ovulation (days 21 to 24). In these studies, delayed implantation was very uncommon; however, when it did occur, a delay in implantation was associated with a higher miscarriage rate, possibly due to a shift in the time of implantation and the concomitant loss of synchrony between endometrium and embryo ( Fig. 9.15 ). This explanation may provide insights into some cases of otherwise unexplained infertility (UI) and unexplained recurrent pregnancy loss (uRPL).
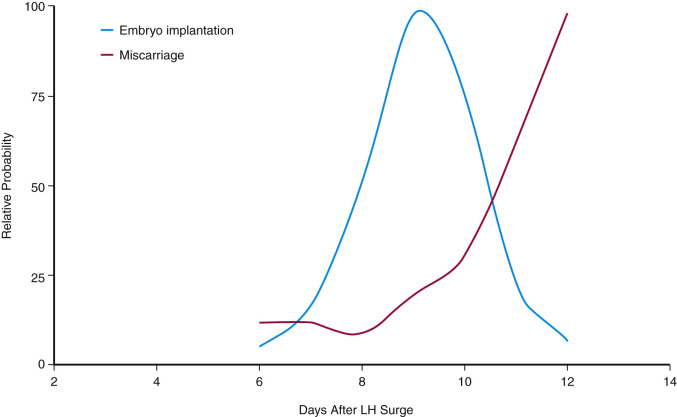
Implantation can be viewed as a highly complex and orchestrated interaction between the maternal endometrium and the newly formed embryo. As depicted in Fig. 9.16 , multiple soluble and membrane-bound factors have been elucidated that facilitate embryo growth, differentiation, attachment, invasion, and avoidance of immunologic rejection. Maternal factors appear to simultaneously permit intrusion while limiting the degree of embryonic invasion into maternal tissue. Many of the embryonic signals or receptors have complementary ligands or coreceptors on the maternal surface. Mimicking of maternal antigens by the invading embryo is a strategy that is also used by the embryo to penetrate the endometrium, without triggering host defenses.
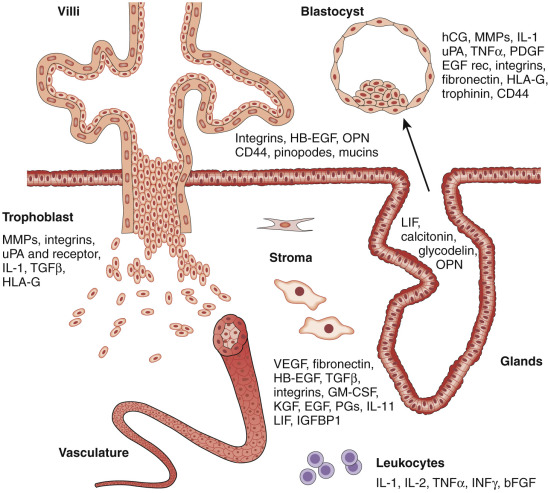
Early Implantation Events
In the event of oocyte fertilization, hCG from the implanting embryo rescues the corpus luteum from apoptosis and regression, maintaining serum progesterone secretion and endometrial integrity. This integrated mechanism, demonstrating the importance of synchrony, creates a window of endometrial and ovarian receptivity that is essential for the success of the pregnancy. Women who are delayed in establishing embryo implantation, beyond the normal window of implantation (7 to 10 days after ovulation), are at increased risk for miscarriage.
The examination of hysterectomy specimens revealed that the first consistent structural changes in the endometrium of early pregnancy are recrudescence or accentuation of glandular secretory activity, edema, and the predecidual reaction. The increased prominence of the vasculature is considered to be a manifestation of increased blood flow, which together with VEGF production (a vascular permeability factor) may account for the associated edema. Endometrial biopsies in a cycle of conception suggest that stromal edema and vascular congestion are the earliest persistent morphologic features of the endometrium of pregnancy.
Within the first weeks of gestation, the endometrium undergoes characteristic changes, in which epithelial cells of the endometrial glands become distended with a clear cytoplasm ( Fig. 9.17 ). Many of the epithelial cells develop enlarged and hyperchromatic nuclei. The enlarged nuclei are polyploid. Parallel channels of endoplasmic reticulum and large mitochondria are abundant in the epithelial cells, and the Golgi complexes have numerous stacked saccules. These changes are commonly referred to as the Arias-Stella reaction . The ultrastructural characteristics of the endometrium are consistent with a hypersecretory state, providing the earliest nutrition and maternal communication with the nascent embryo. As blood flow to the placenta requires vascular remodeling that occurs later, this uterine milk from the endometrial glands provides direct and essential sustenance for the early fetus.
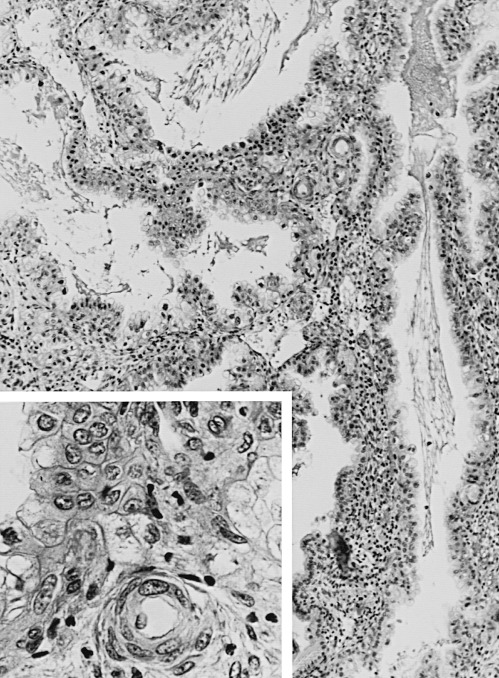
The Arias-Stella reaction has an irregular distribution in the uterus and is present in about 50% of the uteri of women with ectopic pregnancies. It could reflect changes in steroid hormone levels, as well as the direct action of hCG on the endometrium, a subject discussed later in this chapter. The endometrium exhibits significant changes in cellular composition that are reflected in the marked alterations in the synthesis and secretion of endometrial proteins. As gestation advances, endometrial glands atrophy and are scarce at term.
The decidua develops with continued exposure of the uterus to progesterone, secreted first by the corpus luteum and later by the placenta. Based on in vitro studies, other factors (including hCG and relaxin) may act synergistically with progesterone to promote decidualization. Because exogenous cyclic adenosine monophosphate (cAMP) in the presence of progesterone represents a sufficient in vitro stimulus to induce stromal cell differentiation into decidual cells, relaxin and hCG likely exert their effects through this second messenger. Extrapolating from studies on the mouse, including findings on knockout animals that have been found to be defective in decidualization, it is possible that AMP, cAMP, HOXA10, HOXA11, IL-11, LIF, Cox-2, and prostaglandins all have a role in the decidual response in humans.
The decidualized stroma represents a tissue that is both permissive and simultaneously a restrictive barrier of trophoblast invasion and placentation; its remodeling is crucial to the morphogenesis of the placenta and the establishment of the uteroplacental circulation. Moreover, the decidualized stroma represents the arena where the fetal semiallograft is exposed to maternal immunologically competent cells. While creating a hospitable environment for trophoblast invasion, the decidua also sets limits on this process to prevent excessive penetration and tissue destruction beyond its bounds.
The plump, polygonal decidual cells are arranged in a cobblestone configuration. The ultrastructural features of the decidual cells—including prominent Golgi complexes, dilated rough endoplasmic reticulum, and dense membrane-bound secretory granules—are characteristic of secretory cells. The histologically distinct cell borders around decidual cells reflect the accumulation of a pericellular matrix ( Fig. 9.18 ). Abundant decidual cell prolyl hydroxylase, an enzyme involved in collagen synthesis, indicates the important role of these cells in extracellular matrix production. There is also an abundance of amorphous components, including high-molecular-weight proteins with voluminous saccharide moieties (e.g., heparin sulfate proteoglycan). Fibrillar collagens are partially broken down and reorganized. Type V collagen epitopes are unmasked; collagen type VI, “stiff” short collagen fibers that bridge other fibrillar collagens disappear from most of the stroma and persist only in association with vessels and the basement membrane of the glands.
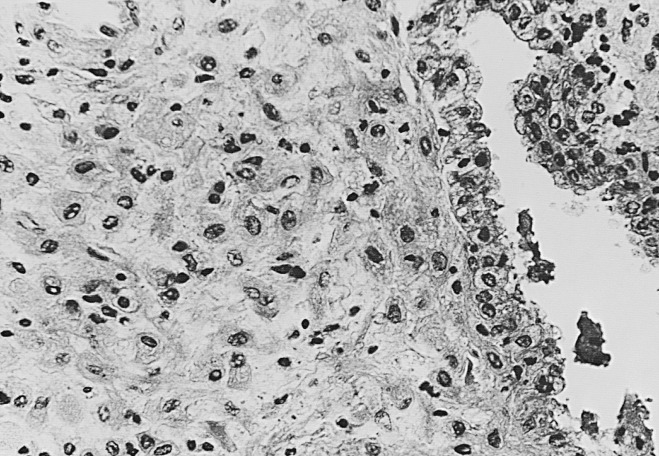
The deposition of a basement membrane-type matrix containing laminin and type II collagens around the decidual cells contributes to the formation of a “looser stroma” that serves as a substrate for the invading trophoblast cells. For example, entactin, a component of this basement membrane-like matrix, promotes trophoblast cell adhesion and migration. The decidual matrix is also a rich source of cytokines, protease inhibitors, protease precursors, and other factors that modulate cell behavior. These are derived, at least in part, from the decidual cells whose secretory products also include IGFBP-1 and TGF-β, which may restrain the invasion of trophoblast cells. Forkhead box OA1 (FOXO1A) has been shown to regulate many of the genes induced during decidualization including IGFPB-1 and prolactin. Ghrelin is an endogenous ligand for the growth hormone (GH) secretagogue receptor and has also been shown to be involved in decidualization of human endometrium as well. Ghrelin, in combination with sex steroids estradiol and progesterone and cAMP, increase both prolactin and IGFBP-1, while ghrelin alone antagonizes the actions of cAMP. Reduced ghrelin has been associated with infertility.
Prior to interaction with the surface epithelium, the blastocyst must hatch from the confines of the zona pellucida. Gradual zona thinning, as well as complete hatching of embryos, can be observed in vitro. The existence of ectopic implantation suggests that the endometrium is not obligatory for this process to be successfully completed. Nevertheless, there may be more subtle regulation of hatching within the endometrial cavity. Although degradation of the zona pellucida is a process controlled by the embryo, inhibitors or agents that induce “zona hardening” may affect the timing of the process.
Work on nonhuman primates demonstrates that mononuclear cytotrophoblasts of the trophectoderm of the blastocyst have fused into syncytia before the attachment of these cells to the endometrial epithelium. Careful histologic descriptions of very early human implantation sites (such as those studied by Hertig et al. ) indicate that the syncytial trophoblast layer of the human embryo comprises the invading front during the first few days of implantation. Thus the consensus appears to be that it is a syncytial trophoblast cell that initially interacts with and adheres to the endometrial epithelium; only after the human embryo is completely embedded in the endometrium do cytotrophoblast cell columns start to stream out of the trophoblastic shell and further invade the uterus. This process starts approximately 1 week after the initiation of implantation and continues well into the second trimester of pregnancy.
The early human implantation sites that have been examined histologically demonstrate that by day 12 after ovulation, the embryo is almost completely covered by endometrium. The endometrial stroma around the implantation site displays the predecidual reaction and is edematous. By the classic histologic criteria of Noyes et al., the endometrium of the implantation site is not that different from the nonpregnant midsecretory phase endometrium. The glands adjacent to the embryo are themselves deflected by invading trophoblasts but maintain the tortuosity and secretion-filled appearance typical for this stage of the menstrual cycle. In vitro observations using human embryos or human trophoblasts have attempted to characterize some morphologic features of the early events of trophoblast-endometrial interactions.
There is growing consensus that initial apposition and attachment is an evanescent and rate limiting step in the initiation of implantation. A receptor-mediated paradigm for embryo attachment and invasion has long been postulated. Numerous endometrial and trophoblast cell-adhesion molecules and associated moieties have been suggested as candidates to serve as attachment receptors. Historically, surface modification of the glycocalyx was a subject of much interest, but more recently the molecules that populate the luminal surface have come under scrutiny. The actual number of receptors and ligands on this surface appears to be somewhat restricted compared with the other cells in the endometrium. As shown in Fig. 9.19 , a limited number of adhesion/attachment components have been suggested. MUC-1 (not shown) is a large glycoprotein that is downregulated on the endometrial surface in most species at the time of implantation, but expressed throughout the menstrual cycle in humans. Nevertheless, debate continues regarding this large glycoprotein as a possible attachment receptor for the human embryo. Other smaller molecules are present and may serve the purpose of initial attachment—including trophinin, integrins, CD44, L-selectins, and HB-EGF.
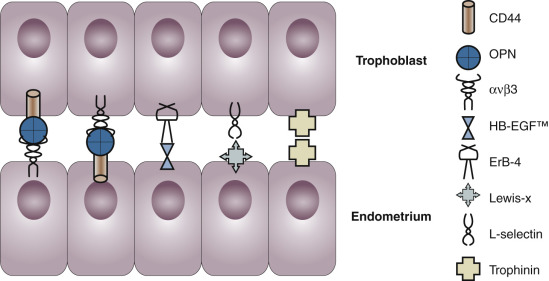
The cascade of events leading to successful implantation likely requires many critical proteins with different functional contributions. A novel cell surface protein called trophinin has been suggested as a homologous pairing partner between trophoblast and endometrium during implantation. The ανβ3 integrin and its ligand OPN are expressed at the time of implantation on the luminal surface of receptive endometrium. Secreted OPN binds to this integrin through an RGD sequence. Since OPN can also bind to the CD44 hyaluronate receptor through non-RGD binding sites, it has been suggested that these serve in a “sandwich”-pairing mechanism at the point of interface. In the human, surface epithelial cells on both the embryo and endometrium express the ανβ3 integrin and CD44. There is also ample evidence that RGD binding is vital to the process of implantation, and peptides containing this sequence reduce implantation efficiency in animal models, including the rabbit and mouse. Thus integrin-mediated adhesion may somehow play a role in successful implantation. More recently it has been suggested that OPN, ανβ3 integrin, and CD44 binding serves to suppress the innate immune system through decay-accelerating factor (DAF)–mediated interference with complement subunit C3, a role that could be critical for the protection of the embryo at the time of initial attachment and invasion.
The transmembrane form of HB-EGF and its receptor ErbB-4 are expressed on the surface of endometrium and on the outer cells of the embryo, respectively. Evidence in both rodents and humans suggests that these molecules could serve as an attachment receptor-ligand pair during implantation. Soluble HB-EGF interferes with this process, perhaps through competitive inhibition. Transmembrane HB-EGF could also play a paracrine role, especially if cleaved from its transmembrane location when the embryo enters the uterine cavity.
Perhaps the most promising mechanism involves that previously elucidated for leukocyte/endothelial interaction. L-selectin and an oligosaccharide ligand are expressed on the blastocyst and endometrial surface in the human, respectively. This type of adhesion reaction between embryo and endometrium at the time of implantation is quite appealing and likely involves integrin mechanisms for embryo invasion, similar to leukocyte intercalation at sites of inflammation.
Evidence from humans illustrates a temporal and spatial regulation of L-selectin ligand expression on the luminal and glandular epithelium. Likewise, each mechanism could be disrupted in infertile women, leading to a failure of implantation. Methods to diagnose and correct such defects may provide new hope for those with otherwise UI or uRPL.
Structural alterations accompany the biochemical changes noted on the surface epithelium. Scanning electron microscopy reveals that the human endometrial epithelium consists of secretory and ciliated cells ( Fig. 9.20A ). The ratio of nonciliated to ciliated cells changes during the menstrual cycle, decreasing in the late proliferative phase and increasing in the secretory phase. In general, estradiol levels correlate directly with the presence of ciliated cells, and withdrawal of estrogen is associated with loss of cilia. The ciliated cells do not undergo surface morphology changes during the menstrual cycle, whereas the secretory cells display significant cycle-dependent surface modifications.
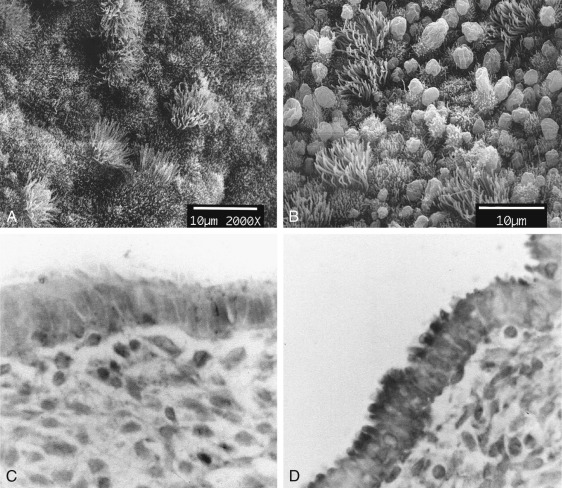
Transient surface specializations of the secretory cells called pinopodes , also known as pinopods or uterodomes, have been a focus of research, because the temporal patterns of expression seem to coincide with the time of maximal uterine receptivity (see Fig. 9.20B ). These surface structures were first identified on the luminal epithelium of rodent endometrium during the limited period (~12 hours), when the uterus is receptive to implantation. They were shown to be involved in pinocytosis; hence the appellation of pinopode (from the Greek “drinking foot”). Similar structures, although with different morphologies, were subsequently discovered in numerous species, including humans; their appearance again generally correlated with the time of implantation.
While it is certain that pinopodes are involved in pinocytosis in the rodent uterus, in vitro studies failed to show that they serve this function in women—hence the suggestion that the structures should be designated “uterodomes” as opposed to “pinopodes.” Mechanisms underlying the formation of pinopodes in the human endometrium have not been elucidated. They may form from the accumulation of membrane components, as a consequence of secretory activity, or from reorganization of the cell cytoskeleton. Some researchers have suggested that they serve to elevate the endometrium above the ciliated cells, providing a platform with the necessary complement of surface adhesion receptors.
The role of pinopodes in human embryo implantation (beyond the temporal correlation between their appearance and the estimated time of nidation) is supported by in vitro studies, demonstrating that human blastocysts implant on human endometrial epithelial cells only in areas bearing pinopodes ( Fig. 9.21 ). Other studies have demonstrated that surface biomarkers are present on the pinopodes. HB-EGF, a molecule implicated as a membrane bound ligand and juxtacrine/paracrine factor important in signaling to the embryo, is on the surface of pinopodes at the expected time of implantation. The ανβ3 integrin and its ligand are also both present on these apical protrusions during the window of implantation (see Fig. 9.20D ). MUC-1 and OPN appear to be on different cell types of the luminal surface, based on electron immunohistochemistry; MUC-1 is present solely on the ciliated cells, while OPN is present on the secretory or pinopode-bearing cells.
The formation of pinopodes appears to be dependent on progesterone, while estrogen causes them to regress. The earlier appearance of pinopodes in controlled ovarian stimulation cycles is correlated with the preovulatory rise in plasma progesterone. Administration of a low dose of the PR antagonist mifepristone on days 14 and 15 of a cycle delays pinopode formation. Studies in the mouse suggest that Hoxa 10, a progesterone-regulated gene, is required for pinopode formation, though pinopodes have been described in both Lif and Hoxa10 null mice. The actual temporal distribution of pinopodes has also been brought into question in the past several years, with several studies showing little correlation with the actual window of implantation. While likely involved in embryo-endometrial interactions, the utility of these structures as markers of uterine receptivity appears limited.
Growth Factors and Cytokines
Various growth factors have been implicated in the dramatic morphologic changes that occur in the endometrium during the menstrual cycle and pregnancy. Among the growth factors whose expression has been demonstrated in the human endometrium and decidua are EGF and EGF-like molecules, including TGF-α and HB-EGF; acidic and basic FGF; IGF-1 and IGF-2; IL-1, IL-11, and IL-6; LIF; the CSF family (CSF-1, CSF-2, and CSF-3); members of the TGF-β superfamily; platelet-derived growth factor (PDGF); tumor necrosis factor-α (TNF-α); and endothelins 1, 2, and 3. Many of these factors have been proposed to play crucial roles in endometrial function and during pregnancy. The endometrium and decidua and embryo/trophoblast have also been shown to express receptors for many of these factors, including the EGF/TGF-α, IGF-1 and IGF-2, IL-1, CSF-1, CSF-2, CSF-3, PDGF, and VEGF. With such a wide array of growth factors, it has become difficult to determine with clarity the role of each factor in endometrial growth and differentiation, or their importance in processes involving maternal embryonic interaction and placental development.
Several growth factors have been shown to exert regulatory effects on the expression of extracellular matrix proteins, their cellular receptors (integrins, selectins, cadherins), and enzymes (MMPs) that also influence cell growth, differentiation, and remodeling.
Leukemia Inhibitor Factor and IL-11
IL-6, LIF, and IL-11 are glycoproteins that belong to the same family of cytokines whose receptors utilize gp130 as a common signaling molecule. LIF acquired its name by its capacity to inhibit the proliferation of a mouse leukemic cell line. LIF is expressed constitutively in the ampullary region of the fallopian tube and in a cyclic fashion in both epithelial and stromal cells of the endometrium, with epithelial expression being greater. The functional LIF receptor, a complex consisting of LIF receptor β (which binds LIF) and gp130 (which mediates signal transduction), is present throughout the menstrual cycle in the luminal epithelium. LIF receptors are expressed by all trophoblast types, particularly the villous syncytiotrophoblast and cytotrophoblasts and to a lesser extent the extravillous trophoblast cells.
Sentinel observations in the mouse have clearly demonstrated that LIF of endometrial origin is crucial for the process of implantation, particularly the decidualization response. LIF-deficient female mice failed to become pregnant or respond to a uterine decidualizing stimulus. However, transfer of their embryos to pseudopregnant, wild-type females resulted in viable pregnancies, as did infusion of LIF into the uteri of LIF-deficient females. The primary action of LIF appears to be on the uterus. However, in mice subsequent placentation is disrupted, perhaps because LIF’s role in modulating trophoblast differentiation and expression of MMPs cannot be executed. Implantation of LIF receptor-deficient human embryos occurs but these offspring suffer from Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome.
LIF mRNA and protein are present in human endometrium, being most abundant in the glandular and luminal epithelium and peaking in the secretory phase of the cycle. The cycle-dependent expression of LIF in human endometrium may be a function of other growth factors, rather than a direct effect of steroid hormones. If implantation occurs, LIF expression by the endometrial glands is downregulated while there is a concomitant increase in LIF expression by endometrial NK and T cells. LIF has been found to enhance human blastocyst formation and to modulate trophoblast differentiation in vitro. LIF levels in uterine flushings rise 7 days after ovulation, reaching a maximum 5 days later. LIF levels in uterine flushings and secretion of LIF from cultured endometrium obtained from patients with repeated implantation failures or UI is decreased, and defects in LIF have been implicated in some cases of RPL.
Leptin has been suggested as a regulator or LIF, through its receptor, OB-R. LIF stimulates hCG in trophoblast. LIF has been implicated in activation of STAT-3; mice homozygous for STAT activation site on gp130 have a defect nearly identical to LIF deficient mice. Suppressor of cytokine signaling protein-3 (SOCS-3) is stimulated by LIF and blocks phosphorylation of gp130 and STATs. In LIF null mice, stromal Cox-2 and epithelial HB-EGF is absent at the site of implantation. Two other EGF family members, AMP and epiregulin (EPR), are also reduced in LIF knockout mice. IL-1 has been shown to induce Cox-2. Prostacylin acting through the PPARγ is essential for decidualizaton. Cox-2 null mice have multiple defects in ovulation, fertilization, and implantation. Together, LIF stimulation of luminal epithelium along with blastocyst may trigger IL-1 that triggers decidual changes.
Mutations in the coding sequence of one copy of the LIF gene were identified in a small number of infertile women (3 of 74 infertile nulligravid subjects), and a presumed polymorphism was found in one of 75 fertile controls and none of 131 nonobstetrical patients. One of the mutations in the infertile group was in the 5′-regulatory region of the LIF gene; the two others were in the coding sequence in a domain thought to be important for LIF binding to its receptor. Unfortunately, the authors did not determine whether LIF levels or bioactivity in uterine flushings or endometrial biopsy material were correlated with genotype. Collectively, however, these observations are consistent with an important role for LIF in human implantation, trophoblast differentiation, or placentation. IL-11 is another member of the IL-6 family that is implicated in the decidual response. As with the LIF knockout mice, female mice lacking the IL-11 receptor α-chain are infertile because of defective decidualization.
IL-11 is present in the human endometrium, and it advances progesterone-induced decidualization of cultured endometrial stromal cells. Both relaxin and prostaglandin E2 increase IL-11 expression. An inhibitor of IL-11 (W147AIL-11) reduces prolactin secretion by ESC in response to RLX and PGE2, suggesting IL-11 is the critical factor in this signaling cascade. Like LIF, IL-11 is an activator of the JAK/STAT signaling pathway through STAT3, which stimulates suppressor of cytokine signaling-3 (SOCS3), a negative feedback mechanism for receptor activity. Ovarian steroids and cAMP differentially stimulate STAT3 and SOCS3, respectively, in vitro, while IL-11 activates both by phosphorylation. Treatment with an antiprogestin increases SOCS3, attenuating IL-11 induced STAT3, suggesting multiple regulatory components, including progesterone. Recent investigations in women suggest that IL-11 and phosphorylated STAT3 are significantly lower in the infertile endometrium compared with controls, while IL-11 receptor and LIF were not different.
Epidermal Growth Factor Family of Growth Factors
The EGF family of growth factors appears to play a major role in uterine development and physiology. The EGF family of ligands is produced by a family of genes, EGF, HB-EGF, AMP, betacellulin (BTC), EPR, tumor growth factor β (TGFβ), and neuregulins (NRG). This EGF family of ligands has the ability to bind and activate one or more of four homologous ErbB receptors via a conserved 60 amino acid “EGF-like” binding domain. These four receptors differ in their activities, and dimerize with each other to further refine the diversity of this growth factor family. Interacting with their receptors, the EGF family acts as autocrine and juxtacrine factors, and some exist as membrane-bound forms that are released by proteolytic cleavage to function in a paracrine or endocrine manner. The proteolytic cleavage is accomplished by cell-surface metalloproteinases, similar to those involved in L-selectin ligand and MUC-1 cleavage, suggesting an important role for metalloproteinase action at the maternal-fetal interface.
The receptors for the EGF ligand family, ErbB1, ErbB2, ErbB3, and ErbB4, are structurally homologous, and specificity of ligand binding is determined by differences in extracellular domain sequences ( Fig. 9.22 ). Molecular studies have determined that these receptors are regulated through the IHH pathway, discussed earlier. The ErbB proteins function as hetero- or homodimers and possibly also as higher order multimers. ErbB2 lacks ligand-binding activity, and ErbB3 lacks functional tyrosine kinase activity. Thus ErbB2 and ErbB3 likely function only as heterodimers, with another ErbB protein supplying the missing function in a dimer.
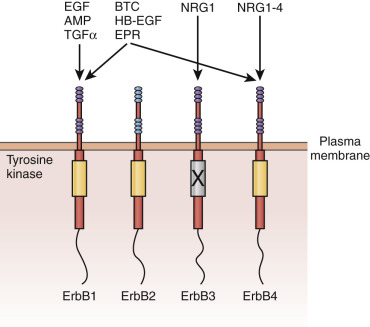
EGF and TGF-α stimulate the proliferation of endometrial stromal cells. The synthesis of fibronectin and vitronectin by epithelial cells is enhanced by EGF. EGF also stimulates stromal cell differentiation and enhances the synthesis of laminin and fibronectin. These growth factors also enhance the morphologic and functional differentiation of decidual cells in vitro. EGF has not been identified in the circulation of cycling or menopausal women, but it has been detected in the serum of pregnant women with peak concentrations occurring in early pregnancy. Binding studies indicate that EGF-R peaks at the time of ovulation (or shortly thereafter) and declines during the secretory phase to a nadir immediately before menses. The binding sites are present on both stromal and epithelial cells, as well as in decidua of pregnancy. Studies using immunohistochemistry suggest that decidualization is associated with an increase in EGF-R. Abnormal EGF and EGF-R activity has been reported in cases of intrauterine growth retardation. Since IHH regulates the EGF receptors, progesterone resistance associated with inflammatory conditions such as endometriosis could account for defects in stromal proliferation and decidua formation noted in this condition. It has been hypothesized that EGF and related molecules play a role in the induction of trophoblast differentiation.
Multiple EGF family members appear to directly participate in the process of implantation. HB-EGF exists as a membrane-anchored precursor (HB-EGF) that gives rise to a soluble processed form. These proteins can bind to two different EGF receptors, HER1/ErbB1 and HER4/ErbB4. HB-EGF mRNA is found in the stroma during the proliferative phase, but in the midsecretory phase, it is also detected in luminal and glandular epithelium. This expression pattern appears to be driven by a combination of progesterone and estrogen.
HB-EGF is postulated to play roles both in adhesion via the membrane-anchored precursor (which binds to HER4/ErbB4 on the apical surface of the trophectoderm) and in stimulating embryo growth. In the rodent, HB-EGF is one of the first cytokines found around the implanting embryo. In the human endometrium, mRNA levels of HB-EGF increase during the secretory phase, reaching a peak at the time of implantation. HB-EGF can mediate attachment of human embryos in an in vitro assay of implantation. HB-EGF also may promote embryonic development. When added to serum-free medium, it increases the number of embryos reaching the blastocyst stage and stimulates hatching. HB-EGF may also participate in paracrine actions within the endometrium. Stromal derived HB-EGF promotes expression of LIF, HOXA10, ανβ3 integrin, and DAF by endometrial cells in vitro. Other members of the EGF family likely play important roles during early pregnancy as well.
Transforming Growth Factor-β Family
The TGF-β family, multifunctional proteins that regulate cell growth and differentiation, includes five dimeric polypeptides encoded by related genes. These growth factor members bind to three cell-surface proteins designated type I, II, and III receptors. The type I and type II receptors are thought to mediate the actions of TGF-β through the SMAD signaling pathway. All three isoforms of TGF-β can be found in human endometrium, including TGF-β1, TGF-β2, and TGF-β3. Null mutant mice bearing TGFβ mutations for each of the three genes produces a distinct phenotype suggesting a separate role for each member of this growth factor family. All three and the type II receptor were identified in all cell types of the endometrium. TGF-β1 and TGF-β3 are found on both epithelial and stromal cells, while TGF-β2 is found primarily on the stroma and increases during the secretory phase. TGF-β3 increases at menstruation and remains high throughout the proliferative phase, while TGF-β1 is maximal at menstruation. Ovarian steroids strongly repress TGF-β2 and TGF-β3 in stroma, but only TGF-β2 is repressed in glandular epithelium. cAMP prevents this inhibition of TGF-β2 by progesterone, while MAP kinase (MAPK) inhibitors differentially stimulated TGF-β2 and TGF-β3. These opposite effects provide insight into the discreet and highly temporal and spatial expression of the TGF cytokines during the menstrual cycle.
TGF-β has profound effects on the extracellular matrix, increasing the synthesis of collagen while decreasing its degradation. In the secretory phase, TGF-β is a potent inhibitor of MMP activity in both stroma (MMP-3) and epithelium (MMP-7), while simultaneously promoting TIMP expression. This cytokine is induced by progesterone, acting in concert with retinoic acid (RA) to maintain these profibrogenic activities. In the event of pregnancy, progesterone acting through TGF-β will maintain the integrity of the endometrium throughout gestation. In lieu of pregnancy, the fall in progesterone releases the MMP inhibitory factors, resulting in programmatic breakdown of the extracellular matrix and subsequent menstruation.
Other members of the TGF-β family are expressed in the endometrium. EBAF (or Lefty) may participate in menstruation process, acting as a potent inhibitor to TGF-β. Antagonism of TGFβ1 by overexpression of EBAF (Lefty; a TGFβ antagonist) reduces the number of implantation sites in rodent models, consistent with human data showing high levels of lefty in infertile women.
Activins are highly abundant in the endometrium. Activin β (βA and βB) is found in the glandular epithelium with peak expression during the secretory phase. Activin A promotes decidualization in vivo, while follistatin opposes the actions of activin. Activin A enhances production of LIF and enhances MMP2 during decidualization. MMP and LIF activity are both crucial for decidualization in rat and primate.
TGF-β1 and TGF-β2 have been reported at the interface between embryo and endometrium, and likely play multiple roles during embryo attachment and invasion. In pregnancy, TGF-β is most abundant in the decidua in the first trimester of pregnancy, where it is thought to restrain trophoblast invasion by promoting trophoblast differentiation away from the invasive phenotype. TGF-β is sequestered in the extracellular matrix, where it can be activated by embryo-derived proteases. TGF-β upregulates the expression of cellular fibronectin by trophoblast cells and induces integrins on the trophoblast that interact with the ECM during invasion, promoting MMP activity. TGF-β induces TIMPs and PAI-1 activity to counterbalance the invasiveness of the trophoblast, and TGF-β is a potent immunosuppressant that may prevent the maternal immune rejection of the fetal allograft. Its actions include suppression of chemotaxis and macrophage and T-cell activity. ADAMTS (a disintegrin and metalloproteinase with thrombospondin repeats) has been thought to play a critical role in extracellular matrix (ECM) remodeling during implantation. IL-1 increases, while the antiinflammatory effects of TGF-β1 decrease, ADAMTS expression. These opposing actions illustrate the balance between factors favoring embryonic invasion and maternal efforts to control this invasion, and reflects the essential role of TGF-β in this process.
Other Growth Factors
PDGF is produced by endometrial stromal cells and also released in the endometrium by activated platelets. PDGF is a potent mitogen that acts on endometrial receptors that are most abundant in the proliferative phase of the cycle.
FGFs encompass a family of growth factors that can stimulate growth of endometrial cells and smooth muscle cells. Acidic and basic FGFs bind to proteoglycans. Because these proteins do not contain secretory signal sequences, they may be most important in the endometrium during menstruation when they could be released from dying cells. Basic FGF is angiogenic, but also stimulates stromal cell proliferation in the presence of progesterone. FGF-7, also known as keratinocyte growth factor, stimulates epithelial cell proliferation. FGF-7 mRNA is expressed at highest level in the late secretory phase endometrium in the stroma; its receptor is most abundant in the glandular epithelium in the late proliferative phase. These findings suggest that FGF-7 is progesterone dependent, whereas its receptor is estrogen sensitive. FGF-8 is a uterine growth factor isolated from bovine uterus, and FGF-9 was recently described in the late proliferative phase of human endometrium.
The IGF system of growth factors encompasses not only IGF-1 and IGF-2 hormones, but also two distinct receptors and multiple different binding proteins that modulate IGF activity. IGF-1 and IGF-2 are mitogenic growth factors that share structural similarities to insulin and are present in the mouse uterus and throughout the menstrual cycle in women. Both hormones are present in the stroma, though the receptors are present in both epithelial and stromal cells. IGF-1 is more abundant in the proliferative phase and may function in epithelial proliferation, while IGF-2 is expressed in a more robust fashion in secretory phase and is thought to be a mitogen for the stroma of pregnancy.
IGF-1 binds to both the type 1 IGF receptor (IGF-1 receptor), structurally similar to the insulin receptor, and the type 2 receptor (IGF-2 receptor) with high affinity. IGF-2 binds the latter with higher affinity.
IGF-1 mRNA is localized by in situ hybridization primarily in the syncytial trophoblast. IGF-2 mRNA has been demonstrated in mesenchymal fibroblasts of the villous core and also expressed in first-trimester and term cytotrophoblasts. The expression of IGFs in the placenta seems to be under the regulatory control of hormones (insulin, human placental lactogen, and estrogens) as well as growth factors, including PDGF. Type IGF-1 receptors have been detected in the placenta during the earliest periods of gestation, and it has been hypothesized that IGFs promote trophoblast proliferation.
TNF-α is a pleiotropic factor that exerts inflammatory, mitogenic, mitostatic, angiogenic, and immunomodulatory effects in a variety of tissues. It is a membrane-bound 14-kDa polypeptide that is derived by proteolytic cleavage from the 26-kDa precursor. Expression of TNF-α mRNA and protein has been demonstrated in human endometrium, decidua, and trophoblasts; its receptors have also been found in all these tissues. TNF-α in the human endometrium is subject to regulation by steroid hormones—namely, estrogens and progesterone.
TNF-α and its receptors (TNF-R) are expressed by trophoblasts during both early and late gestation. There is differential expression of the two genes encoding TNF-R, thus allowing some regulation of TNF-α activity. In cultured human chorionic cells, TNF-α affects cellular fibronectin secretion. Scientists have hypothesized that, along with other endometrial and trophoblast factors, TNF-α controls trophoblast adhesion and invasion, and alters the integrin expression pattern in endometrial stroma.
Colony-stimulating factors are a family of three cytokines, CSF-1, CSF-2, and CSF-3, that were initially identified by their ability to stimulate hematopoietic stem cells to form colonies in semisolid culture media, with CSF-1, 2, and 3 promoting proliferation and differentiation of macrophage, granulocytes plus macrophage, and granulocytes, respectively. The original observation that short-term cultures of human placental tissue produced large amounts of CSFs has led to further study of these factors in trophoblast and, more recently, in endometrium. Despite growing enthusiasm for clinical therapy with CSFs to improve implantation, human data on their normal endometrial expression and function are minimal.
CSF-1 gene expression essential for normal fertility in mice and expression of CSF-1, its receptor, are found in the endometrium, decidua, and placenta, with a higher CSF-1 expression in the secretory phase and decidua than in the proliferative phase. The CSF-1 receptor is highly expressed by extravillous trophoblasts in the cell columns that anchor the placenta to the uterus, with nearby decidual cells expressing CSF-1, consistent with in vitro data demonstrating CSF-1 effects on trophoblast proliferation and differentiation. The phenotype of the op/op (osteopetrotic) mouse that lacks CSF-1 argues against an essential role for this factor, as the observed reduced fertility and smaller litter sizes are due, in large part, to an ovulation defect, though placental abnormalities are evident in a CSF-1 knockout. Thus, despite production of CSF-1 by the endometrium, presence of CSF-1 receptor on the trophoblast, and clear evidence of CSF-1 effects on trophoblast function, a definitive role for endometrial CSF-1 remains to be established.
CSF2 and its receptor are also produced by both the placenta and endometrium. CSF-2 has been shown to increase proliferation of trophoblast cells in vitro and murine csf2−/− placentae demonstrate altered morphology and gene expression; CSF2 has been shown to have a potent effect on prenatal programming in the bovine. As is the case for CSF-1, a significant role for CSF-2 in human reproduction remains unclear.
CSF3 is monomeric glycoprotein, originally characterized as a factor promoting production, differentiation, and function of granulocytes. Although CSF3 and its receptor are also produced by endometrium and placenta, the details of this expression are not well described. Despite the lack of information, CSF3 has been shown to be an effective treatment in recurrent miscarriage and implantation failure, each in a single randomized trial. Although confirmatory studies are needed, initial data suggest that targeting maternal CSF3 receptor may be an important therapy for some infertile couples.
Human Chorionic Gonadotropin
In vitro studies demonstrate that the addition of follicle-stimulating hormone, luteinizing hormone (LH), hCG, thyroid-stimulating hormone, and free β subunit have an effect on human reproductive tract tissues—including the stimulation of prolactin production, enhancement of decidualization, and myometrial relaxation. The presence of LH/hCG receptors in the fallopian tube, myometrium, and endometrium has also been reported. However, the transcripts detected are smaller than those encoding the gonadal LH/hCG receptors; the proteins are also smaller, detected as 50- to 60-kDa molecules as compared with the observed receptor molecular weights in gonadal tissue (83 to 95 kDa). Thus the extragonadal LH/hCG receptors appear to be truncated, evidently lacking extracellular domains, but still retaining the capacity to signal after binding LH and hCG. The primary signal transduction cascade initiated by the truncated endometrial LH/hCG receptors may not involve the classical cAMP/protein kinase A system as the primary pathway, but rather the mitogen-activated protein kinase pathway or the activation of prostaglandin synthesis.
Although extragonadal LH/hCG receptors have been proposed to play various roles in the reproductive tract, they are probably most important in the context of pregnancy where high levels of hCG are present. Thus hCG could be an important embryonic signal in the bidirectional dialogue between conceptus and uterus. The best evidence that extragonadal LH/hCG receptors have an important role in primate reproduction is derived from the study of the baboon, where administration of recombinant hCG causes alterations in both epithelial and stromal cells. Preliminary studies in women indicate that hCG can also affect the human endometrium in vivo, including prevention of endometrial apoptosis. In clinical trials, hCG support has been shown to decrease miscarriage, perhaps by this mechanism of CL rescue and avoiding loss of CL support to the nascent pregnancy.
In the baboon, the response to intrauterine administration of hCG by osmotic minipump over a 4-day period starting on day 6 after ovulation includes the formation of an epithelial plaque, hypertrophy of the surface epithelium, and rounding off of the glands characteristic of pregnancy in this species. Glycodelin expression in the endometrial glandular epithelium is enhanced, and α-smooth muscle cell actin is expressed in the stroma. In vitro, hCG inhibits stromal cell apoptosis and stimulates decidual changes—reflected by increased IGFBP-1 expression as well as increased expression of COX-2. As previously noted, in vitro studies have shown that the glycoprotein α subunit stimulates production of prolactin, another uterine secretory product. Activation of myometrial LH/hCG receptors in vitro causes myometrial relaxation, which could facilitate nidation in vivo. The response to hCG was blunted in animals with endometriosis.
In vivo studies have demonstrated a direct effect of hCG on endometrial histology and receptivity. Using intrauterine microdialysis in the human, it was shown that administration of hCG significantly reduces IGFBP1 and M-CSF expression in human after postovulatory day 10, while LIF, VEGF, and MMP9 were all dramatically increased, suggesting that hCG is an important modulating factor during early pregnancy. In vitro studies, using hCG coated beads, have demonstrated an induction of trophinin on the endometrial epithelium when IL-1 is present, suggesting a mechanism for enhanced embryo/endometrial interactions at the maternal-fetal interface. LIF and its receptor are upregulated in response to hCG treatment in vitro. As an endocrine factor, hCG likely has independent effects on a wide variety of endometrial genes, independent of direct trophoblast interactions.
Prostanoids and Other Lipids
The role of prostaglandins in the implantation process has long been suspected because of their effects on the vascular system and association with inflammatory processes (see Chapter 4 ). The fact that mice deficient in COX-2 display abnormalities in the implantation process—particularly the early decidual response—is consistent with this notion. Evidence suggestive of a role of prostanoids in human implantation includes the presence of COX-1 and COX-2 in the human endometrium (mainly the glandular epithelium) during the presumptive implantation period ; an examination of prenatal use of nonsteroidal antiinflammatory drugs (NSAIDs), which inhibit COX enzymes, indicated an increased risk of miscarriage in users of aspirin and other NSAIDs. One of the key prostanoids involved in implantation is thought to be prostacyclin, a ligand for the peroxisome proliferators-activated receptor delta (PPARδ), a nuclear receptor family member expressed in subluminal stromal cells in the rodent uterus. This transcription factor is implicated in the implantation process as well.
Another lipid implicated in implantation is the arachidonate derivative known as arichidonylethanolamide or anandamide, a ligand for the cannabinoid receptors. Anandamide, referred to as an endocannabinoid, binds to cannabinoid receptors CB1-R and CB2-R, which are expressed in the preimplantation embryo and in the reproductive tract. The embryo is enriched in CB1-R, and in the blastocyst, the expression of this receptor is most abundant in the trophectoderm. Uterine anandamide levels in the mouse are reduced at the time of implantation and are highest at interimplantation sites. Endocannabinoids at low levels accelerate trophoblast differentiation, but at high levels inhibit trophoblast differentiation and arrest embryo development. Tetrahydrocannabinol and synthetic agonists of the cannabinoid receptors have similar effects. Thus it has been postulated that endocannabinoids play an important role in controlling the synchrony of embryo development for implantation in rodents. Anandamide is present in human reproductive tract fluids, and high anandamide levels are associated with in vitro fertilization (IVF) failures.
Immunology of the Endometrium
The uterus is an immunologically privileged organ: it can accommodate tissue invasion by immunologically semiforeign placental cells, yet maintain mucosal immune defenses against ascending foreign organisms, and provide a system to efficiently clear the endometrial detritus that results from menstruation (see Chapter 13 ). Remarkably, the endometrium also uses mechanisms of acute inflammation during normal, hormonally regulated physiologic processes, including menstruation and embryo implantation. These acute inflammatory episodes are quickly resolved, avoiding the consequence of scarring and dysfunction. Despite the description of critical active processes to resolve inflammation in other tissues and the clear relevance to endometrial physiology and pathophysiology, mechanisms that resolve endometrial remain largely unstudied.
The complex requirements of uterine immunity and tolerance use overlapping and redundant mechanisms dependent on both innate and adaptive branches of the immune system. Endometrial immune processes, as with other uterine functions, but unlike those for other mucosal immune sites, are markedly influenced by cyclic and pregnancy-specific changes in sex steroid concentrations and possibly by hCG.
The endometrium is populated by bone-marrow derived immune cells, as well as endometrial epithelial and stromal cells that demonstrate immune functions. As is the case for many epithelial cells, endometrial epithelium express members of the Toll-like receptor family (TLR2 to 6, 9, and 10), which detect pathogen products and trigger a cellular response to these “foreign” molecules, including peptidoglycans from Gram positive bacteria (TLR2), lipopolysaccharide from Gram negative bacteria (TLR4), and unmethylated CpG islands found in bacterial DNA (TLR9). The endometrium also produces host defense molecules, defensins, as well as cytokines and chemokines. Uterine lymphoid and myeloid cells play roles in tissue defense, immune modulation, angiogenesis, and tissue remodeling. These cells are present in the fallopian tubes, uterus, and cervix, with the fallopian tubes and uterus containing a higher proportion of leukocytes than the cervix and vagina.
The endometrial innate and adaptive immune systems are regulated by steroid hormones. For example, progesterone induces a local Th2-type cytokine response in the uterus, which includes an increase in IL-4, IL-5, and IL-15 and downregulation of the IL-13 receptor α2, which is a negative regulator of the antiinflammatory cytokine, IL-13, and powerful inhibitor of the Th2 response. The Th2 response is believed to counter proinflammatory processes in the endometrium that could lead to rejection of the embryo. Steroid hormone-directed alterations in endometrial chemokine production influence the trafficking of blood leukocytes in the reproductive tract. Further, actions of progesterone are important in the overall immune suppressive phenotype adopted by the receptive endometrium.
During the secretory phase, there is a profound recruitment of leukocytes into the endometrium starting in the perivascular locations around spiral arterioles and glandular epithelium. The progesterone-induced alteration in endometrial cytokine/chemokine production contributes to this recruitment. Cytokines IL-1, IL-11, IL-15, LIF, and TGF-β regulate trafficking of leukocytes to the endometrium. IL-15 recruits NK cells into the endometrium, and IL-15 knockout mice lack uNK cells. Locally acting prostaglandins (PG), including PGE along with VEGF, modulate vascular permeability. Cyclooxygenase-2 (COX-2), a rate limiting enzyme that regulates the biosynthesis of PGE2, is critical to implantation in the mouse, and in animal models, PG are required for initiation and maintenance of decidualization. Blockade of COX-2 prevents decidualization in mice and clearly plays a role in endometrial function surrounding pregnancy, with reduced COX-2 associated with implantation failure and uRPL.
An immunotolerance maternal immune response is essential for the acquisition of endometrial receptivity and the success of pregnancy. Factors that support a more suppressed immune environment, including the recruitment of T regulatory cells (Tregs) and a shunting away from a proinflammatory, Th1/Th17 responses are central to our understanding of infertility and pregnancy loss associated with various inflammatory conditions such as endometriosis, as discussed later.
Leukocytes and Lymphocytes
There are striking menstrual cycle dependent changes in the immune cell population of the endometrium. Neutrophils, the most abundant leukocytes of the immune system, are rare in the normal endometrium until the perimenstrual phase, when they accumulate and account for 6% to 15% of the total cell number. Eosinophils are also rarely found in the normal endometrium until the perimenstrual phase, when they also accumulate—usually in aggregates—and show evidence of activation, as revealed by the extracellular location of eosinophil cationic proteins. Macrophages are present in the endometrium throughout the cycle, increasing in number from the proliferative to the menstrual phases. Immediately prior to menstruation, the number of macrophages in the functional layer of the endometrium is equivalent to that of neutrophils.
Mast cells expressing tryptase (a characteristic of mucosal mast cells) and chymase (a characteristic of connective tissue mast cells) are found in the human endometrium. In the functionalis, mast cells are positive only for tryptase, while those in the basalis express both enzymes. Degranulation of these cells may initiate activation of MMPs as a result of tryptase and chymase action on proMMP-1 and proMMP-3.
The endometrial lymphoid system has a distinctive composition and activity. There is good reason to believe that endometrial T cells are activated in situ, as judged by their expression of antigens that are characteristic of the activated state; these antigens include the MHC class II molecules HLA-DR, HLA-DP, and HLA-DQ, and very late antigen 1. CD3+ T cells represent only 1% to 2% of the lymphomyeloid cells detectable in the human endometrium. They are present throughout the cycle in aggregates in the basalis, as well as singly throughout the stroma and in intraepithelial sites. The number of these cells increases prior to menstruation. The ratio of CD4 + T helper lymphocytes to CD8 + cytotoxic T cells in the endometrium is inverted compared with peripheral blood. The latter have cytolytic activity during the proliferative phase, with much reduced secretory phase activity. Few B cells and plasma cells are present in the human endometrium.
Leukocytes make up between 15% and 30% of the cellular mass of the endometrium. The complex network of cytokines produced directly or indirectly in response to progesterone likely is involved in leukocyte recruitment to the endometrium. Progesterone (P) is essential for implantation, regulating complex and coordinated biochemical interactions in the endometrium that supervise the controlled invasion of the fetal allograft, including direct involvement in the establishment of immunologic tolerance. The actions of P are mediated in part through RA and TGFβ and PPAR-γ, with a collective profound influence on CD4+ leukocyte differentiation. The actions of P (and RA and PPAR-γ) are disrupted in certain gynecologic disorders, including endometriosis, leading to alterations in the endometrial phenotype, contributing to infertility and early pregnancy loss. Normal eutopic endometrium hosts leukocytes in both stromal and intraepithelial locations, and an immunotolerant environment appears essential for successful implantation. Recent evidence suggests that maternal immune responses are critical for the acquisition of endometrial receptivity and that immune cells can directly regulate endometrial epithelial receptivity and associated gene expression. Macrophages have been shown to regulate uterine epithelial gene expression in the mouse, suggesting a mechanism for observed differences in eutopic endometrial gene expression differences, including progesterone resistance seen in endometriosis. Conversely, inflammatory cytokines in peritoneal fluid of endometriosis might alter the differentiation pathways of monocytes to a more hostile phenotype.
T-Regulatory and TH-17 Cells Regulate Endometrial Receptivity
As discussed in Chapter 13 , the immune cells can be classified as belonging to the innate branch, including monocytes, macrophages, dendritic cells (DC), neutrophils, basophils, mast cells, and lymphoid NK cells. The adaptive branch immune cells include the T and B cells, which generally require innate immune cells for activation and establishment of immunologic memory. BM-derived cells are known to traffic to endometrium, via steroid-regulated chemokine production. Under the proper circumstances, DC and Treg cells increase at the implantation site, along with uNK cells that interact with the invading placental cells to both direct and limit trophoblast invasion. BM-derived decidual cells ultimately determine pregnancy outcomes. Monocyte-derived leukocytes and uNK cells comprise up to 20% to 40% of all endometrial cells in the secretory phase and are critical to the establishment of endometrial receptivity. A loss of function may account for UI and uRPL, or in women with minimal and mild endometriosis.
Adaptation of innate immunity is required for pregnancy and survival of the semiallograft. This immunotolerance is mediated in part by T-regulatory (Treg) cells. Treg cells are suppressors of inflammatory immune responses that keep the immune system in control. In the mouse model, Treg cells proliferate and are activated in the uterus in response to paternal antigens and nonspecific hormonally regulated factors. In contrast to Tregs are Th17 cells, a proinflammatory T cell subtype associated with multiple autoimmune and inflammatory conditions including colitis, multiple sclerosis, and rheumatoid arthritis. IL-9 induces Th17 cell differentiation, and in mouse models of inflammatory disease, attenuation of IL-9 decreases Th17 cells and reduces IL-6 producing macrophages, contributing to Th17 differentiation. This balance in T-cell differentiation may ultimately define whether an endometrium is receptive to the fertilized oocyte and whether a pregnancy will be successful.
Uterine Natural Killer Cells
The uterine NK cell, also known as the granular lymphocyte, is a unique member of the lymphoid lineage found in the endometrium. These are round cells that characteristically have bilobed or indented nuclei and a pale cytoplasm containing acidophilic granules. They are a specialized subset of NK cells based on their expression of cell surface antigens (CD3 − , CD16 + , and NCAM/CD56 bright ). This pattern is different from blood NK cells, which have a phenotype of CD56 dimand CD16 + .
Uterine NK cells are among the most abundant lymphomyeloid cells in the perimenstrual endometrium. Very few are present in the proliferative phase endometrium; they accumulate during the secretory phase, at which time they comprise 15% to 25% of the cells in the endometrial stroma. It has been postulated that the dynamic changes in uterine NK cell number during the cycle are determined by changes in endometrial prolactin production, which is then controlled by ovarian steroid hormones. This notion is based on the known roles of prolactin as an immunomodulator and the correlation between endometrial prolactin levels and NK cells.
The NK cells persist in the decidua during the first trimester, when they represent 70% of the decidual leukocyte population. Their close association with stromal cells has led to the suggestion that they have an important role in initiating and maintaining decidualization. In a nonfertile cycle, the NK cells that amass during the secretory phase undergo programmed cell death.
When activated IL-2 in vitro, the NK cells become competent to kill malignant cells (and some normal ones) through the release of cytotoxic proteins like perforin. From the late proliferative phase on, the NK cells express cytotoxic activity and are thought to play a role in protecting the endometrium against infection. The cells have also been proposed to modulate trophoblast invasion during implantation and placentation because of their abundance in the decidua during the first trimester.
Although in vitro studies document the killing activity of cytokine-activated NK cells, there is little evidence for destruction of trophoblast cells in vivo. Thus any restraining activity that NK cells exert on invading trophoblast cells appears to be through a noncytotoxic mechanism that may involve secreted cytokines, including colony-stimulating factor-1 (CSF-1), IL-1, LIF, and interferon-γ. The apparent in vitro and in vivo resistance of trophoblast cells to death may be explained by the fact that trophoblast cells express a nonclassical and nonpolymorphic major histocompatibility complex (MHC) class I antigen, HLA-G; NK killing activity is also suppressed by endometrial stromal cells.
In the transgenic mouse line TgE26, which lacks NK cells, abnormal implantation sites are found. Fetal demise follows, in association with changes in uterine arterioles suggestive of arteriosclerosis and hypertension. These histopathologic changes are reminiscent of preeclampsia in humans. In contrast, mice lacking IL-15, and consequently, circulating and decidual NK cells, are fertile but show a thickening in uterine vessels. These findings collectively implicate NK cells in vessel remodeling, a role consistent with their expression of angiogenic factors.
Innate Lymphoid Cells
In addition to NK cells, other uterine innate lymphoid cells (ILCs) have been recently examined in cycling human endometrium and decidua. Three types of ILCs have been described, but these study identified only type 3 ILCs in human endometrium. Type three ILCs seem to play a role in in-gut immunity and mucosal homeostasis, and these cells play an important role in innate immune colitis, suggesting potential roles in endometrial inflammatory regulation. However, an important role for these cells in endometrial physiology or pathology remains speculative.
Regulation of Endometrial Immune Cells
The changes in lymphomyeloid cell populations in the endometrium, particularly their accumulation in the premenstrual phase, appear to be the result of recruitment from the peripheral circulation and intraendometrial proliferation. The accumulation of migratory cells is directed by chemoattractant cytokines, chemokines, and the expression of intercellular adhesion molecules that attach leukocytes to the endothelium in preparation for extravasation and trafficking through the endometrium. Expression of these molecules changes during the menstrual cycle, at least partly under the influence of steroid hormones.
The expression of several chemokines has been documented in the human endometrium including fractalkine (CX3CL1), RANTES (CCL5), IL-8, MCP-1 (CCL2), MCP-2 (CCL8), and eotaxin (CCL11). These chemokines bind to receptors on leukocytes, promoting the appearance of molecules that mediate adhesion to the endothelium, extravasation, and chemotaxis along the concentration gradient of the chemokine, thus targeting specific leukocyte types to specific endometrial compartments. A repertoire of cell adhesion molecules expressed in the endometrium, including ICAM-1, ICAM-2, VCAM-1, E-selectin, and PECAM, specifies the recruitment and location of leukocyte and platelet accumulation. ICAM-1 is present in the functionalis in the menstrual phase; ICAM-2 is restricted to the vascular endothelium and does not appear to change during the cycle, VCAM-1 and E-selectin appear in the upper layer of the functionalis in the secretory phase, and the platelet endothelial cell adhesion molecule PECAM is abundant in the stroma during the menstrual phase.
Evidence for leukocyte proliferation in the endometrium includes the expression of Ki67 and incorporation of BrdU (both proliferation markers) by NK cells with a marked increase in the secretory phase. The uterine NK cell proliferation may be driven by progesterone-regulated stromal factors as CD56 + cells proliferate in vitro in the presence of progesterone-treated endometrial tissue.
LIF is associated with a shift in Th1 to Th2 shift that is required for normal pregnancy and is defective in recurrent abortions in mice. Maternal T cells increased LIF expression in response to paternal antigens and trophoblast, and reduced response was associated with poor reproductive outcomes. Progesterone is an antiinflammatory immunomodulator in pregnancy and through induction of LIF and other cytokines that support trophoblast invasion.
LIF and another biomarker of endometrial receptivity, ανβ3 integrin, are coexpressed in mouse and human endometrium, and like ανβ3 integrin, disturbances in LIF expression are associated with multiple reproductive pathologies. While recent reports have linked these two important biomarkers, their coregulation has only recently been demonstrated. Absence of both integrin and LIF is associated with decreased pregnancy success in IVF. Injection of PF from women with endometriosis reduced both LIF and ανβ3 integrin expression in the mouse uterus. Both LIF and ανβ3 integrin are positively regulated by paracrine actions of P (HB-EGF), and negatively regulated by inflammatory immune cells in the eutopic endometrium associated with endometriosis and inflammatory tubal disease. LIF has antiinflammatory actions regulating cytokine action, protecting against septic shock in the mouse model, whereas ανβ3 integrin has been shown to interact with decay accelerating factor (DAF) and OPN, which together block complement activation as part of innate immunity. T cells from women with RPL secrete less LIF than normal women, and defective LIF is associated with decreased Th2 cells and RPL.
Complement System
The human fallopian tube, endometrium, and cervical mucosa express components of the complement system. The activation of the third (C3) and fourth (C4) component of complement induces chemotaxis of inflammatory cells, enhances phagocytosis, and mediates cell lysis. This system of natural immunity must be tightly regulated so that it can target foreign organisms and cells while avoiding untoward tissue damage. This is especially important during early pregnancy, when the process of implantation might be disrupted. Complement activation is regulated by DAF (also known as CD55), which inactivates the C3 convertase enzymes that activate C3; and by membrane cofactor protein (MCP, also known as CD46), which serves as a cofactor for factor I-mediated degradation of activated C3 and C4.
A third protein, present only in rodents and named complement regulator DAF (Crry), has DAF- and MCP-like activities. Crry controls deposition of activated C3 and C4 on the surface of autologous cells. Fetal demise due to complement deposition and placental inflammation was observed in mice lacking Crry, reflecting unchecked activation of the complement system. Complement activation appears to be an essential event in pregnancy loss associated with antiphospholipid syndrome, and can be prevented by administration of heparin. These observations suggest that control of the complement system is essential for fetomaternal tolerance against attack by the innate immune system.
In the endometrium, complement component C3, factor B, and DAF are present in the glandular epithelium, and their expression is upregulated in the secretory phase. MCP is expressed in the glandular epithelium throughout the menstrual cycle. Complement receptor type 1 is present in the stroma during the secretory phase, complement receptor 2 is not detectable, and complement receptor 3 is associated with infiltrating leukocytes in the luteal phase. The cyclic changes in C3, factor B, and DAF expression suggest progesterone regulation. In model human endometrial epithelial cell culture systems, however, steroid hormones did not alter DAF expression, but heparin-binding epidermal-like growth factor (HB-EGF) did increase it. Thus the steroid hormone effects on expression of some of the complement system proteins may be indirect, perhaps acting through the stromal compartment.
Members of the integrin family are now thought to be involved in the regulation of C3, as part of the protective proteins that prevent the cascade of complement activation. DAF or factor H may interact with these cell surface receptors (including the ανβ3 integrin and perhaps CD44) through binding to OPN to limit C3 activation by digesting bound C3b. The ανβ3 integrin, OPN, and DAF all appear synchronously at the time of implantation in human endometrium and may serve as a primary safeguard against complement activation at the time of embryonic attachment, thus avoiding destruction of the early pregnancy by the host’s defenses.
Antimicrobial Peptides
Epithelial cells of the female reproductive tract elaborate antimicrobial peptides that presumably guard against ascending infection. These peptides coat the epithelial surface and enter into the cervical mucus. Among the antimicrobial peptides expressed other than complement are lactoferrin, lysozyme, secretory leukocyte protease inhibitor (SLP1), and defensins. The α-defensins are elaborated by leukocytes and epithelial cells, the β-defensins by epithelial cells. Defensin-5, and α-defensin, is expressed in the upper half of the stratified squamous epithelium of the vagina and ectocervix, and in columnar epithelial cells of the fallopian tube and endometrium. β-defensin-1 and -2 and SLP1 are produced by endometrial glandular epithelial cells. β-defensin-2 expression is increased by proinflammatory cytokines, and defensin-5 is also evidently upregulated by inflammation. Defensin-5 and SLP1 levels in the endometrium also fluctuate during the endometrial cycle, being highest in the secretory phase.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree