Abstract
The function of the hypothalamo-pituitary-testicular-accessory organ axis is to ensure normal androgenization (including embryonic, infantile, pubertal, and adult sexual maturation), male sexual behavior, and reproductively competent sperm output. Thus the system is important for both the health of individuals and preservation of humankind. All elements in the system acting together as an integrative regulatory network are critical for male reproductive health. Dysfunction may result in ambiguous genitalia, sex reversal, pubertal delay, eunuchism, impaired spermatogenesis, and reduced systemic androgen exposure. Furthermore, partial deficiency of reproductive hormones may contribute to some of the features of male aging, impair recovery from protracted critical illness, and induce visceral adiposity with insulin resistance. This chapter reviews the hypothalamo-pituitary-testicular axis, its components, and their joint regulation. Where practicable, emphasis is on newer discoveries in humans. Hormonal methods to regulate male fertility and to limit age-associated frailty constitute examples of how knowledge of male reproductive physiology is being translated into clinical practice.
Keywords
Testis, hypothalamus, pituitary, prostate, seminal vesicles, testosterone, estradiol, gonadotropin-releasing hormone, luteinizing hormone, kisspeptin
Physiology of the Male Gonadal Axis
- ◆
Gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (Te), and estradiol (E 2 ) are the major ligands (hormones) of the male gonadal axis.
- ◆
Pulsatile secretion of these hormones allows feedforward (stimulatory) and feedback (inhibitory) signaling through their cognate receptors to maintain androgenization, male sexual behavior, and reproductively competent spermatogenesis.
- ◆
The master regulator of the male gonadal axis was long thought to be the GnRH neuron, but recent investigations now implicate upstream kisspeptin, neurokinin B, and the endogenous opioid peptide, dynorphin (KNDy) neurons as the master regulator, and have better defined how KNDy neurons are regulated by steroidal and nonsteroidal signals.
Overview: Ensemble Nature of Reproductive System
Male reproductive hormones comprise the hypothalamic decapeptide, GnRH, pituitary gonadotropins, luteinizing and follicle stimulating hormone (LH and FSH), testicular steroids, Te and E 2 , and putatively systemically active gonadal peptides like inhibin B. Integration requires repeated incremental and decremental feedforward (stimulatory) and feedback (inhibitory) signaling among neural and hormonal components of the axis ( Fig. 12.1 ). The negative-feedback system can be exploited to regulate male fertility by suppressing spermatogenesis with exogenous sex hormones. Gonadotropin and Te deficiency can result in infertility or subfertility, impaired sexual maturation, reduced muscle and bone mass, insulin resistance, visceral fat accumulation, diminished erythropoiesis, libido, potency, and general well-being.
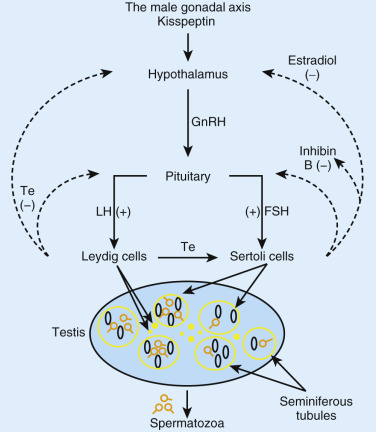
Essential Roles of Gonadotropin-Releasing Hormone and Upstream KiSS1 Neurons
In the adult, specialized neurons (~1200 in number in the human) located in the arcuate-nucleus of the mediobasal hypothalamus secrete GnRH in a pulsatile fashion into a portal microvasculature system (see Chapter 1 ). GnRH pulses stimulate gonadotropes located in the anterior pituitary to secrete LH and FSH. In the embryo, migration of GnRH neurons from the olfactory placode into the upper diencephalon is essential to establish the anatomic proximity required for effectual hypothalamo-pituitary signaling. Several genes ( ANOS1, FGFR1, and PROK2 ) are critical for both olfactory-bulb morphogenesis and proper GnRH neuronal migration. ANOS1, FGFR1, and PROK2 are located on chromosomes X, 8, and 3, and respectively encode anosmin-1, fibroblast growth-factor (FGF) receptor 1, and prokineticin 2. Inactivating mutations of any of these genes, including FGF8, which is the ligand for the FGF receptor 1, and less commonly neuroembryonic LHRH factor (NSMF), cause Kallmann syndrome. The syndrome is characterized clinically by hyposmia or anosmia and isolated gonadotropin deficiency. Inheritance patterns are respectively X-linked, autosomal dominant, and recessive for defects in anosmin, FGF-receptor 1, and prokineticin or its receptor. Recently identified mutations in the chromodomain helicase DNA binding protein-7, which cause CHARGE syndrome, may also present with Kallmann syndrome and idiopathic hypogonadotropic hypogonadism. Additional pleiotropic genes, which cause reproductive and nonreproductive phenotypes, manifest mutations identified in at least 25 separate loci. Next-generation sequencing and other modern methods will continue to unveil novel mutations. These mutations will ultimately need to be functionally validated experimentally or bioinformatically.
Pulsatile GnRH release is both intrinsic to clusters of GnRH neurons and essential to stimulate LH secretion over the long term. Continuous GnRH delivery downregulates gonadotrope GnRH receptors and signaling pathways, which forms the basis for treating prostatic cancer and precocious puberty with long-acting GnRH agonists. Episodic GnRH secretion begins during fetal development when GnRH neurons first populate the mediobasal hypothalamus. A second surge of high-amplitude LH pulsatility emerges in infant boys during the first several months of age. GnRH secretion declines during childhood only to be reinstated at markedly higher amplitude and slightly higher frequency in puberty.
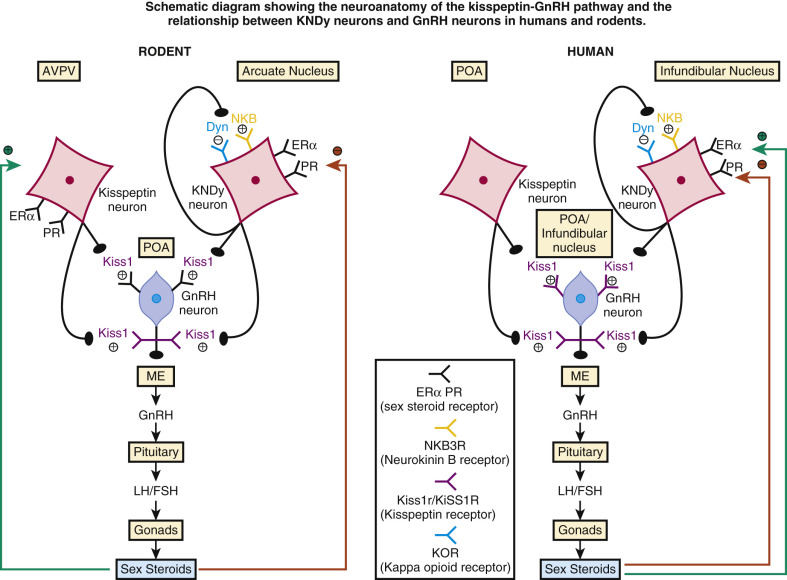
The kisspeptin-kiss1R system controls the timing of puberty, and it may also regulate GnRH secretion in other epochs of life. Kiss1R (formerly known as GPR54) is a G-protein coupled receptor expressed on GnRH neurons. KiSS-1 is the gene that encodes kisspeptin-121, which is proteolytically cleaved to a 54 amino-acid peptide named kisspeptin-54 (also known as metastin) and kisspeptin-14 and 13, and possibly further modified by endopeptidase 24.15. Kisspeptin-54 is the natural ligand for the receptor Kiss1R. Inactivating mutations of either KiSS-1 or Kiss1R in mice and of KiSS1 in men result in normosmic hypogonadotropic hypogonadism, thus establishing that kisspeptin and cognate receptor are necessary upstream activators of GnRH neurons. Mutations in the genes TAC3 and TACR3, which encode the peptide neurokinin B and its receptor, respectively, also result in hypogonadotropic hypogonadism. Furthermore, specific neurons (KNDy neurons) in the arcuate nucleus of the hypothalamus, which coexpress kisspeptin, neurokinin B, and the endogenous opioid peptide, dynorphin, are critical for sex-steroid feedback regulation of GnRH secretion. Proximate control of kisspeptin neurons and Kiss1R receptors by sex steroids and neurotransmitters is under intensive study ( Fig. 12.2 ). The stimulatory role of neurokinin B and the inhibitory role of dynorphin autosynaptically synchronize KNDy neurons, resulting in coordinated Kiss1 secretion, which ultimately increases both the frequency and amplitude of pulsatile secretion of GnRH and LH. Table 12.1 highlights some of a myriad of GnRH regulators, whose site(s) of action require more precise definition. Regulators include seasonal, appetitive, anorexigenic, stress-associated, and anabolic (trophic) factors.
| Ligand | Role | References |
|---|---|---|
| GnRH * | Autoinhibit (or stimulate) | |
| Kisspeptin | Activate GPR54 receptor on GnRH neurons | |
| Galanin, neuromedin B | Stimulatory of GnRH release | |
| Glutamate | Activate N -methyl- d -aspartate receptor | |
| Norepinephrine | Excitatory (beta-1 receptor) or inhibitory (alpha-1 receptor) | |
| GABA † | Inhibit or stimulate | |
| Nitric oxide | Stimulatory | |
| Dopamine | Repressor or activator | |
| Neuropeptide Y | Activate or inhibit via distinct receptor subtypes | |
| CART ‡ | Stimulate | |
| Opiatergic peptides | Repress, for example, dynorphin | |
| Prolactin | Inhibit via cognate receptor | |
| Calcitonin gene-related peptide | Inhibit | |
| Ciliary neurotrophic factor | Stimulate | |
| Leptin, orexin A/B | Stimulate GnRH outflow fasting | |
| Somatostatin | Inhibit GnRH neurons | |
| Serotonin | Inhibit or stimulate (via 5HT-1A or 2C and 4) | |
| Tachykinins, neurokinin B and substance P | Stimulatory |
* CRH, AVP, and α-MSH inhibit, whereas melanin-concentrating hormone stimulates, GnRH release.
† GABA, Gamma-amino butyric acid acts via a type A or type B receptor.
Sex steroids, acting in part via estrogen-receptor alpha, regulate KiSS1, neurokinin B, and dynorphin gene expression in a brain region-specific manner. Estrogen and androgen repress both KiSS1 and dynorphin in KNDy neurons in the arcuate nucleus, thereby inferably transducing sex-steroidal inhibition of GnRH outflow. This is not true in the anteroventral periventricular nucleus, which directs “positive feedback” by estrogen in female rodents (see Fig. 12.2 , left panel). The latter site contains few KiSS1 neurons in the male. Other neurotransmitters may be important, since the administration of antihypertensives (particular alpha-adrenergic blockers, selective serotonin-reuptake inhibitors, gamma-aminobutyric acid agonists, and dopamine) can influence LH secretion. However, whether such effects are direct or indirect with respect to KiSS1 and GnRH neurons remains largely unexplored.
Differential Control of Luteinizing Hormone and Follicle-Stimulating Hormone Secretion
GnRH preferentially stimulates FSH secretion in childhood and LH secretion in infancy, puberty, and adulthood. Like GnRH, kisspeptin stimulates LH release twofold more than FSH release in healthy men. The putative bases for these distinctions include feedforward and feedback factors. With respect to feedforward, slower GnRH pulse frequencies prior to puberty favor gonadotrope biosynthesis of specific FSH-β over LH-β subunits. In addition, the dimeric glycoprotein, activin A, may stimulate FSH secretion in the prepubertal rat and nonhuman primate. At a second level of feedforward, blood-borne FSH acts on Sertoli cells in spermatogenic tubules to drive inhibin B and E 2 secretion, and LH acts on Leydig cells in the testis interstitium to cause Te production. There is some indirect evidence that FSH can also increase Te production, possibly via Sertoli cell-secreted nonsteroidal factors, but the effect is quantitatively small. In relation to resultant feedback, pubertal elevations in inhibin B and E 2 concentrations would tend to restrict FSH responses to GnRH. Elevated Te concentrations would likewise tend to repress the rise in LH concentrations. The fact that the latter does not occur suggests muting of Te’s feedback and/or strong central GnRH drive. Both mechanisms are inferable from mathematical feedback analyses performed in transpubertal cohorts of boys.
Te’s negative feedback on LH is mediated primarily but not exclusively via local aromatization to E 2 . Significant feedback occurs at the pituitary level. Sex steroids repress GnRH secretion, but inhibition is not likely to be direct, because GnRH neurons do not express the androgen receptor (AR) or estrogen receptor-α (ERα). Although ER-β is detectable and might enhance Ca 2+ -dependent synchrony of GnRH neurons, transgenic silencing of AR or ERα rather than ERβ is needed to elevate LH and Te concentrations in the adult animal. These data favor a feedback role of KiSS1 neurons, which express both AR and ERα. In addition, nongenomic actions of Te and E 2 transduced via membrane-dependent signaling may contribute to feedback in some species.
Gonadotropin-Releasing Hormone and Gonadotropin-Releasing Hormone Receptors
More than 23 different GnRH peptides exist among species. Human GnRH decapeptide exists in at least two forms designated GnRH I and GnRH II, cloned initially in mammals and chickens, respectively. The genes encoding these peptides are located on chromosome 8p11–21 and 20p13, respectively. Rapid and slow GnRH half-lives in men are about 2.6 and 5.2 minutes. GnRH I is the predominant agonistic peptide regulating gonadotropin secretion in humans. In animals GnRH II seems to stimulate sexual arousal and courting behavior, whereas GnRH III (lamprey) induces significant FSH release. Truncational mutation of the GnRH I gene occurs in the hpg/hpg mouse, but has not been described in man.
At least 23 GnRH-receptor types have been cloned to date. GnRH receptors are calcium-dependent G-protein coupled receptors (GPRs), which control the activity of intracellular heterotrimeric G proteins by promoting guanine-nucleotide exchange and activation of adenylyl cyclase, transmembrane Ca 2+ influx, or intracellular Ca 2+ mobilization. GPRs contain seven transmembrane segments that form α-helices connected by three extracellular and three intracellular loops. Receptor activation causes a conformational change in the associated G protein α-subunit, promoting the release of GDP and binding of the activator nucleotide, GTP. The GTP-bound α-subunit dissociates from the receptor leaving a stable βγ-dimer. Dissociated subunits modulate cellular signaling as proximate messengers in the cyclic AMP-protein kinase A (α subunit) and phospholipase C-protein kinase C (βγ dimer) pathways. High intrahypothalamic concentrations of GnRH may mediate autoinhibition via a G i subunit.
Two distinct GnRH receptors have been identified in man. GnRH I receptor is the hypophyseal receptor coupled to LH and FSH synthesis, which is encoded by a gene located on chromosome 4. Two splice variants of the GnRH I receptor exist. Both GnRH I (secreted) and GnRH II (brain) peptides can activate the GnRH I receptor. The GnRH II receptor gene is located on chromosome 1, but is silenced in the human, chimpanzee, cow, and sheep. GnRH regulates neurotransmission and sexual behavior in the rodent via the GnRH II receptor. Truncational, nonsense, and missense mutations in the GnRH receptor I gene or homeobox genes (LHX3) can lead to nonexpression, inactivation, misfolding, and misrouting of nascent receptors, and thereby autosomal recessive hypogonadotropic hypogonadism without anosmia. Activating mutations of the GnRH receptor could result in isosexual precocious puberty, but have not yet been identified. Other non-GnRH signals (e.g., IGF-I, galanin, activin A, inhibin B, follistatin, cytokines, gonadotropin-inhibitory hormone) may potentiate or inhibit GnRH action in the pituitary in animal models.
Gonadotropins and Cognate Receptors
Gonadotropins comprise lutropins (LH and human chorionic gonadotropin, hCG) and follitropin (FSH) (see Chapter 2 ). Gonadotropins and thyroid-stimulating hormone (TSH) are oligosaccharide-modified dimeric proteins with a molecular mass of 30 to 40 kDa. All four corresponding genes are expressed in the human pituitary gland, albeit minimally in the case of hCG. Each contains a common α subunit and a hormone-specific β subunit. Subunits are associated by noncovalent interactions, but cysteine residues (10 in the α subunit and 12 in the β subunit) permit disulfide linkages within subunits. The α subunit is encoded by a single gene localized at 6q12.21. Mutations of this gene have not been identified, possibly because simultaneous loss of all glycoprotein hormones may be lethal in utero. The α subunit gene is larger than the β subunit gene because of a noncoding exon 1 and a long first intron, but the translated proteins are comparable in size. Beta-subunit genes are located on chromosomes 19q13.32 (LH/hCG cluster) and 11p13 (FSH). The human LH/hCG cluster consists of one LHβ and six hCGβ genes and pseudogenes. Glycosylation and terminal sialylation or sulfation via critical N- and O-linked sites within α (two sites) and β (one site in LH, two in FSH and TSH) subunits influence in vivo bioactivity, receptor binding, and metabolic clearance. In particular, four O-linked glycosylation linkages present in an extended C-terminal portion of hCGβ (but not LHβ) increase intact hCG half-life to 24 to 30 hours, compared with 1 hour for LH, and augment biopotency by limiting hCG-receptor dissociation.
Exonic polymorphisms and mutations have been described for each gonadotropin β subunit (hCG, LH, and FSH) gene. In mice, knockout of LHβ results in decreased production of Te and fewer elongated spermatids. One polymorphism of LHβ, termed variant LHβ, occurs in as many as 30% of some Northern European and Australian aboriginal populations, and contains two amino-acid transversions (Ile15Thr and Trp8Arg) and a supernumerary consensus glycosylation site (Asn13-Ala-Thr) reminiscent of hCGβ. Variant LH is more biopotent, but has a shorter in vivo half-life than wild-type LH putatively, due to resistance to peptidases or impaired sialylation and sulfation of terminal oligosaccharides. A single nucleotide polymorphism (SNP) in the promoter of the FSHβ gene (−211G>T) is present in about 15% of Baltic men. The T allele is associated with lower serum FSH and semen sperm concentrations, and smaller testis volume. In contrast, frank mutations of the FSHβ gene are rare in men. There are only three reported cases, each being associated with undetectable FSH and azoospermia and one with hypoandrogenism.
The LH/hCG receptor is a G-protein coupled receptor that is encoded by an 11-exon gene located on chromosome 2p21. The LH/hCG receptor and the FSH receptor (as well as the TSH receptor) are characterized by a large extracellular leucine-rich-repeat hormone binding domain and possess a common internal peptide sequence near the C-terminal part of the extracellular domain that is critical for signal transduction. Unlike the FSH receptor, the lutropin receptor expressed is widely outside the gonad including in the brain, leading to speculative extragonadal actions of LH or hCG. Men with inactivating LH-receptor mutations present with male pseudohermaphroditism and a phenotype ranging from micropenis and hypospadias to complete feminization, in each case without an extragonadal phenotype. Activating mutations of the LH/hCG receptor become manifest as gonadotropin-independent (familial) male sexual precocity or testotoxicosis. LH-receptor polymorphisms have also been described (such as Asn291Ser and Asn312Ser) in association with undermasculinization and Leydig cell hypoplasia. A rare splice variant (deleted exon 9) is marked by inhibitory properties. Thus structural analyses of interactions among LH receptor, ligand, and relevant G proteins should lead to targeted ligand discovery.
The FSH receptor is also a G-protein coupled receptor, but is encoded by a 10-exon gene located on chromosome 2p21. The crystal structure has been determined, revealing a handclasp interaction between the receptor and FSH (follitropin) that orients the glycoprotein hormone perpendicular to the long axis of the ligand-binding domain. The follitropin receptors appear to be expressed exclusively on Sertoli cells located within the blood-testis barrier formed by tight junctions. For this reason, FSH is believed to regulate spermatogenesis primarily. A few controversial reports exist of FSH-receptor expression elsewhere, such as on spermatogonia or skeletal osteoclasts. Replication of these preliminary findings is required.
Polymorphisms of the FSH receptor (e.g., FSHR Asn680Ser) occur. The Ser allele is associated with lower testis volume and FSH concentrations. However, the effect of this allele is small (−1.40 mL of testis volume) and was detected only after stratifying for the FSHβ (−211G>T) polymorphism. These data show a newly recognized interplay between the FSHβ and FSH receptor genes under physiologic conditions. Inactivating mutations of the FSH receptor in five men were associated with subfertility (three cases) and fertility (two cases), with phenotypic variations ranging from oligozoospermia to euspermia. Such data suggest that very low receptor activity is sufficient for spermatogenesis and/or that other endocrine or intratesticular factors may compensate to varying degrees. An activating mutation of the FSH receptor was reported, but functional significance has not been corroborated.
In young men, FSH seems necessary for the formation of spermatogonia type B and pachytene spermatocytes, and LH/hCG for round spermatids. In contrast, LH is the dominant agonist of steroidogenesis.
Testicular Steroidogenesis
- ◆
Te is biosynthesized from cholesterol sequentially through reactions that are catalyzed by cytochrome P450 enzymes.
- ◆
Te is the most important local and systemic androgen produced by the testis.
- ◆
Aromatization diversifies and 5α-reduction amplifies the action of Te.
Te is the most important local and systemic androgen produced by the testis. Te concentrations are similar by race and ethnicity, but decline with increased abdominal visceral fat and with age. In contrast, African American men have higher E 2 and sex hormone-binding globulin (SHBG) concentrations than their white counterparts. Androgens are crucial for male reproduction and general health. Te triggers the development of male secondary sexual characteristics at puberty, maintains adult sexual behavior and function, and drives late stages of spermatogenesis independently of its metabolite 5α-dihydrotestosterone (DHT). During fetal development Te is essential for differentiation of the internal and DHT the external male urogenital system. Inactivation of 5α-reductase type I in mice is lethal to the embryo (putatively due to unopposed brain estrogen toxicity), whereas inactivation of 5α-reductase type II in mice and men leads to male pseudohermaphroditism (perineoscrotal pseudovaginal hypospadias, hypoplastic prostate tissue, and decreased male-pattern balding and beard growth). Despite low or absent DHT in such XY individuals, Te is sufficient to ensure wolffian-duct differentiation (viz., epididymides, vasa deferentia, seminal vesicles). Other androgens, such as the reduced metabolite, 5α-androstane-3α,17β-diol, are important for fetal sexual maturation in marsupials, but their roles in primates are unknown.
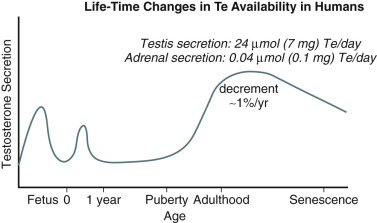
Leydig cells are located in the interstitial compartment of the testis, which allows secretion of Te and E 2 into seminiferous tubules, gonadal lymphatics, and venules. Fetal Leydig cells and maternal hCG are responsible for an intrauterine peak in Te secretion at 12 to 14 weeks of gestation. This peak is critical for male urogenital-tract organogenesis. Te production declines during the remainder of in utero life as fetal Leydig cells begin to degenerate. Augmented pulsatile LH secretion and neonatal proliferation of Leydig cells result in a second peak of androgen production at 2 to 3 months of postnatal age. The neonatal peak is putatively important for imprinting masculine behavior at least in animals. Fetal Leydig cells undergo apoptosis but are slowly replaced as mesenchymal stem cells populate the interstitium, and differentiate into initially immature Leydig cells that secrete 3α- and 5α-reduced androgens rather than Te. Further division and differentiation occur in puberty, when mature (adult) Leydig cells secrete large amounts of Te ( Fig. 12.3 ). Beginning in young adulthood and progressively thereafter, total Te concentrations fall gradually by 0.6% to 1.1% annually, resulting in a net decrement of 35% to 50% from the young-adult maximum by the eighth decade in life.
Te is synthesized from cholesterol through sequential reactions that are catalyzed by cytochrome P450-containing complexes (see Chapter 4 ). A defect in any step results in variably severe male pseudohermaphroditism (46XY infant with undermasculinization of the external genitalia). Five major steps are involved, namely cholesterol C20,22-desmolase (CYP11A cholesterol side-chain cleavage), 3 β-hydroxysteroid dehydrogenase/Δ -isomerase (3 β-OHSDH), C17,20-desmolase/17α-hydroxylase (CYP17A), and 17 β-hydroxysteroid dehydrogenase type III ( Fig. 12.4 ). Cytochrome P450 refers to a 450-nm light-absorbing heme-binding peptide sequence that is critical for stoichiometry. The enzymatically rate-limiting step in steroidogenesis is encoded by CYP11A, which converts cholesterol to pregnenolone in the inner mitochondrial membrane. However, the kinetically rate-limiting step is delivery of LDL-, membrane-, and cholesteryl ester-derived free cholesterol to the inner mitochondrial leaflet by steroidogenic acute regulatory (StAR) protein. LH activates StAR rapidly via posttranslational modification and slowly via increased gene transcription. Combined responses ensure both immediate and sustained access of cholesterol to mitochondrial side-chain cleavage system.
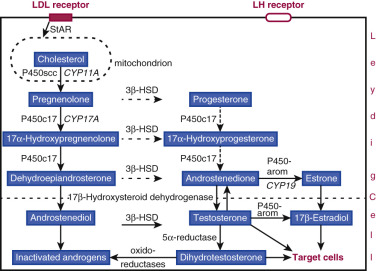
Pregnenolone leaves mitochondria and enters endoplasmic reticulum, where subsequent enzymatic modifications occur. Pregnenolone (or its 3 β-reduced metabolite, progesterone) undergoes sequential cleavage and hydroxylation via CYP17A-encoded C17,20 lyase/17α-hydroxylase (a single enzyme with dual functions), which constitutes the androgen-committing step. The product is a weak ketosteroid, androstenedione, which must be converted to the potent hydroxysteroid, Te, via 17β-hydroxysteroid dehydrogenase type 3.
In men, gonadal steroidogenesis accounts for more than 95% of Te production, with the remainder produced by the adrenal gland and extraglandular conversion of the weak androgenic precursors, dehydroepiandrosterone and androstenedione, to Te. Te production in young men averages 15 to 30 µmol (4 to 9 mg) per day. Estimates are consistent when determined by infusion of stable or radioactive isotopes and recent noninvasive analytical methods. Te secretion is determined genetically and environmentally. For example, men convert more Te to E 2 by peripheral aromatization and more androstenedione to Te via 17β-hydroxysteroid dehydrogenation than women. In addition, Te concentrations differ between ethnic Chinese men living in an Asian environment compared with a Western environment. Combined factors also may explain some populational differences in 5-α reduced metabolites and prostate volume.
Intratesticular regulation of Leydig cells is complex, and not yet fully understood. All three of spermatogonial, peritubular myoid, and Sertoli cells exert effects on Leydig-cell steroidogenesis. Central nervous system and spinal-cord pathways that innervate the testis further modulate testicular steroid production, particularly in the settings of inflammatory stress, alcohol intoxication, and central adrenergic modulation. Certain proinflammatory cytokines have been directly implicated. Interleukin-2 induces androgen depletion presumptively through enhanced feedback inhibition of LH secretion in older men, whereas tumor necrosis factor α reduces GnRH secretion, KISS1R expression, and kisspeptin-induced depolarization in primary fetal hypothalamic cell cultures.
Aromatization, 5α-Reduction, and Inactivation of Testosterone
Untransformed Te exerts potent anabolic effects. Te also functions as a prohormone, which undergoes conversion to other steroids, such as E 2 and DHT. Converted steroids can mute or amplify, and thus diversify, Te’s actions through ligand-receptor and coactivator specificities ( Fig. 12.5 ). In particular, DHT binds ERα negligibly, whereas E 2 binds AR with an affinity one order of magnitude less than that of Te. Te directly stimulates AR, but with a potency 2- to 30-fold less than that of DHT, depending upon the target tissue. Thus, by being reduced or aromatized, Te may oppose or potentiate the actions of E 2 on certain genes.
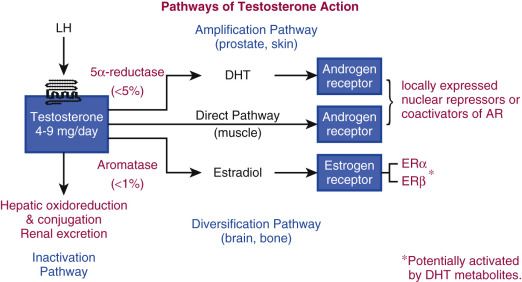
Estradiol acts via two primary (and multiple variant) ERs, whereas Te and DHT act via a single AR. Heterogeneity of tissue responses is presumably conferred via cellular differences in the expression of AR and important corepressors and coactivators. The existence of tissue-selective modulators of nuclear AR action confers a basis for designing pharmacologically selective AR modulators (SARMs) to target anabolism in osteoblasts, erythropoietic precursors, stellate cells in skeletal muscle, and the brain, while exerting little or no effect on adipocytes or the prostate gland. A subset of SARMs may be suitable for use in male contraception. Compared with Te, DHT is more potent due to both higher affinity for and slower dissociation from AR. DHT is considered a pure androgen in that it is nonaromatizable. Only 4% of blood Te is converted to DHT, less than 1% is transformed to E 2 , and approximately 2% to androstenedione (see Fig. 12.5 ). Although systemic transformation is minimal, tissue-specific conversion of androstenedione to Te and of Te to DHT or E 2 may be critical in organs expressing AR or ER. For example, systemic Te administration elevates intraprostatic DHT concentrations by providing substrate for local 5α-reductase type II, whereas exogenous DHT lowers prostatic DHT concentrations by suppressing LH and thereby Te secretion. Other hormones such as vitamin D, glucocorticoids, and progesterone also modify 5α-reductase under pharmacologic conditions.
The CYP19 gene, located on chromosome 15q21.1, encodes the enzyme aromatase, which converts Te to E 2 and androstenedione to estrone. Aromatase is widely distributed in the testis (Leydig cells, Sertoli cells, spermatid lineage), bone, brain, pituitary, liver, intestine, skin fibroblasts, adipocytes, and breast stromal cells. Systemic aromatization predominantly (>60%) proceeds in extraabdominal adipose tissue. The coexistence of CYP19 and ER in the brain, pituitary, and testis suggests that locally produced E 2 is physiologically important in these key sites. Evidence favors a role for E 2 and ERα in stimulating libido and mating frequency, especially in animals, inhibiting testis descent into the scrotum by repressing the INSL-3 gene, opposing germ-cell apoptosis in older animals, decreasing LH secretion, inhibiting Leydig-cell growth and differentiation, maintaining efferent spermatogenic ductule water reabsorption and endocytosis, and stimulating male breast anlagen ( Table 12.2 ). Moreover, transgenic silencing and rare human mutations of CYP19 or ERα have unmasked important roles for E 2 in bone-mineral density, fat-deposition patterns, appetitive behavior, and feedback on LH and FSH. Experiments in normal men using pharmacologic agents that block aromatase activity have confirmed many of these findings. Splice variants and polymorphisms of CYP19 exist, which might modify the risks of prostatic cancer or osteoporosis.
| Action | Mechanism * |
|---|---|
| ↓ GnRH gene transcription | GnRH neuronal ERβ and kisspeptin ERα |
| ↓ Gonadotropin concentrations | ↓ LH and FSH pulse size |
| ↓ GnRH-stimulated LH secretion | ↓ GnRH efficacy at pituitary level |
| ↓ Te biosynthesis | ↓ CYP17A, StAR and LH receptor |
| ↓ Testis descent | ↓ insulin-like factor 3 in Leydig cells via ERα |
| ↓ Spermatogenesis | ↓ FSH by feedback in adult |
| ↓ Epididymal and seminiferous-tubule fluid absorption | Putatively via ERα, which maintains aquaporin-1 expression |
| ↓ Germ-cell apoptosis | Older male animal |
| ↓ Leydig cell proliferation | Fetal and puberal testis |
| ↑ Male sexual arousal and aggressive behavior | Neonatal brain ERα pathways |
Two isoforms (type I and type II) of 5α-reductase are present in the human, encoded by separate genes located on chromosomes 5p15 and 2p23. Type I is found mainly in the skin, liver, and brain, and type II primarily in male accessory sex glands, liver, and brain. The isoenzymes share approximately 50% structural peptide homology. Polymorphisms of both isoenzymes have been reported, some of which (e.g., V89L) alter enzymatic activity or correlate with sperm output (A49TT and V89LV). Genetic variations in DHT synthesis or catabolism were thought to contribute to interindividual variability in spermatogenic suppression by Te in male contraceptive regimens. However, prospective assessments of this postulate did not verify the original finding.
Te and DHT are inactivated in the liver, kidney, and prostate by mixed-function oxidoreductases (e.g., 3α- and 3β-hydroxysteroid dehydrogenases) or glucuronidases (conjugation reactions). Three-alpha reduction of DHT decreases its affinity for AR by a factor of 100,000-fold. The oxidoreduced and conjugated metabolites are lost into urine and bile, respectively. Certain metabolites of DHT, such as 5 alpha-androstane-3 beta, 17 beta-diol may mimic E 2 action via ERβ or modulate neurotransmission (see Fig. 12.5 ).
Progesterone
Although gonadotropes and KiSS1 neurons express nuclear progesterone receptors, there is no established role of progesterone in adult human male reproductive physiology. Exogenous progesterone and synthetic progestins can suppress gonadotropin output in vivo through the hypothalamic progesterone receptor, inhibit Te biosynthesis in vitro by downregulating Leydig-cell LH receptors, and may directly impair spermatogenesis. Reduced metabolites of pregnenolone and progesterone exert inhibitory effects on neurotransmission in experimental models, but the relevance of these actions in men is unknown.
Sex-Steroid Receptors and Sex Hormone-Binding Globulin
- ◆
Steroidal action is further modulated through polymorphisms of the AR, the presence of more than one estrogen receptor, and binding to SHBG.
- ◆
Binding to SHBG mediates steroid-hormone retention and transportation in plasma, restricts metabolism and inactivation, and thereby modulates action.
- ◆
Recent data show that current binding assumptions, analytical methods, and models of binding are adequate for SHBG concentrations within the normal male range, despite advances in the measurement of SHBG and the modeling of binding.
Sex-steroid hormones are lipophilic, thus allowing passive transmembrane diffusion into the cell before interaction with specific high-affinity nuclear receptors. Whether active transmembrane transport also occurs is possible but unproven. Ligand-receptor binding prompts dissociation of chaperone proteins (heat shock protein [Hsp] 90 and immunophilin Hsp 56), receptor dimerization, and interaction with distinct palindromic DNA sequences known as steroid-responsive elements. Typical motifs consist of hexanucleotide sequences arranged as inverted repeats that are separated by three nonconserved base pairs (GGTACAnnnTGTTCT). The classical genomic mode of action is represented by this pathway, in which steroid-hormone receptors function as ligand-activated transcription factors.
Circumstantial evidence in many cell systems suggests that steroids may also act through nongenomic membrane-associated signaling mechanisms. In general, nongenomic pathways are inferred when responses are (1) too rapid to arise by nuclear effects; (2) evident in cells lacking sex-steroid receptors; (3) persistent in the presence of inhibitors of transcription and protein synthesis; and/or (4) elicited by steroids bound to substances that preclude entry into the cell. When defined in this manner, membrane-associated pathways trigger calcium flux, cyclic AMP generation, and extracellular-regulated or mitogen-activated protein kinases. However, the precise molecular relationship between putative membrane and known nuclear receptors is not clear.
Androgen Receptor in Testes
The AR is encoded by a gene located on the X chromosome. Splice variants of the AR exist, but the full-length isoform is the predominant or only form expressed in many tissues, including male reproductive organs. In the testis, AR expression is prominent in early-stage Sertoli cells associated with stage III of the spermatogenic cycle, and to a lesser extent Leydig and myoid but not germ cells. Murine knockout models indicate that AR activity is essential in Sertoli and Leydig cells for fertility, due to AR’s role in spermatogenesis and steroidogenesis, respectively. In contrast, functional AR in peritubular myoid cells is not critical for reproduction. Other tissues expressing AR include prostate, seminal vesicles, skin, fat, liver, all three of smooth, skeletal, and cardiac muscle, brain, pineal gland, bone, and cartilage. Leydig-cell Te stimulates AR in spinal motoneurons, thereby activating gonadal descent into the scrotum via muscles in the outer layer of the gubernaculums (“rudder”) of the testis. A Leydig cell-derived peptide, insulin-like factor (INSL3), albeit insufficient without Te, is necessary for development of gubernacular ligaments, and prevention of cryptorchidism. INSL3 is a relaxin-like ligand, which signals by the LGR8/GREAT receptor.
Inactivating mutations of AR result in the androgen-insensitivity (testicular-feminization) syndrome in the genetic male, and are the most common cause of male intersex. Genotype and phenotype are strongly correlated, since mutations that increasingly impair receptor function result in more marked androgen insensitivity. A regularly updated electronic database of AR mutations is available ( http://www.androgendb.mcgill.ca/ ).
Polymorphisms that modify AR function have been described, such as polymorphic polyglycine (GGC) and polyglutamine (CAG) repeats. Increasing polyglutamine-repeat length is associated with decreasing AR transcriptional activity, impaired spermatogenesis or subfertility, and genital abnormalities in some but not all studies. Polyglutamine-repeat length may contribute to nonuniform suppression of sperm output during male hormonal contraception. Selected nuclear coregulators of AR could be targets of tissue-specific androgen-receptor modulators.
Estrogen Receptors
The genes encoding ERα and ERβ have been mapped to chromosomes 6 and 14, respectively. There are striking similarities in both the DNA-recognition and ligand-binding domains of the expressed receptor proteins. ERα is expressed in pituitary, brain, kisspeptin (but not GnRH) neurons, fat, skin, bone, seminal vesicles, Leydig cells, round spermatocytes, and spermatids, whereas ERβ is detectable in gonadotropes, kisspeptin and GnRH neurons, prostatic epithelium, and Sertoli, Leydig, and spermatogonial cells in several mammals. Male ERα-knockout mice exhibit elevated LH concentrations due to impaired negative feedback, enhanced Leydig-cell steroidogenesis, and oligo/azoospermia associated with efferent ductule occlusion. In contrast, ERβ-disabled mice maintain normal LH and Te concentrations, have decreased germ-cell apoptosis in later adulthood, Leydig-cell hyperplasia, and normal fertility. ERα and ERβ can act individually or as heterodimers, and can exert opposing or synergistic effects upon target genes, thus creating signaling diversity (see Fig. 12.5 ). Splice variants and polymorphisms are described for both ER subtypes, some of which may be linked to reduced sperm output and infertility. Environmental xenoestrogens and phytoestrogens could in principle influence spermatogenesis further in genotypically susceptible individuals. Estradiol may also activate membrane-bound receptors, including G protein coupled ER-1 (previously called GRP30), which is linked to cAMP, and others coupled to extracellular-regulated kinases or Ca 2+ . Some but not all E 2 actions depend upon estrogen-response elements (ERE). For example, ERα and ERE are together necessary to maintain male sexual behavior, whereas either ERα or non-ERE-mediated pathways can suppress LH and Te secretion in mice.
Progesterone Receptor
A single gene located on chromosome 11q22–23 encodes two progesterone receptor isoforms (termed A and B) through alternative transcriptional initiation sites. The isoforms exert reciprocal effects via interactions with selected DNA motifs, defined as progesterone-responsive elements. Progesterone receptors are expressed widely, including in KiSS1 neurons, gonadotropes, breast, brain, and adipose tissue. One recent report identified the progesterone receptor in the rodent testis, but whether progesterone receptors are expressed in the primate testis remains debated. Although physiologic actions of progesterone remain to be elucidated in men, pharmacologic amounts of progesterone and progestins suppress gonadotropin output, which has utility in the design of male hormonal contraception.
Sex Hormone-Binding Globulin: Steroid Transporter and Putative Ligand
SHBG in the male primate is expressed in the liver, adrenal cortex, prostatic alveoli, epididymides, and seminiferous tubules. Infusion of SHBG acutely reduces the metabolic clearance of Te and E 2 in male monkeys. Crystallographic studies show that SHBG is a homodimer structurally similar to the pentraxin family of proteins. Each monomeric subunit contains one hydrophobic steroid-binding pocket, as well as tandem laminin G-like domains near the N terminus that are critical for dimerization. Homodimerization is induced by the presence of steroid ligand and stabilized by calcium ions. Both potential steroid-binding regions in the dimer are occupied by Te. SHBG contains several glycosylation sites, which give rise to multiple posttranslational isoelectric isoforms, and by common variants (polymorphisms) or mutations that alter glycosylation sites resulting in inefficient secretion or metabolic clearance of secreted SHBG. Mutagenesis studies suggest that glycosylation sites in the C-terminus of SHBG may be important for cell-surface recognition. Current binding assumptions, analytical methods, and models of binding appear adequate for SHBG concentrations within the normal male range, despite advances in the measurement of SHBG and the modeling of binding.
Te, E 2 , and progesterone bind SHBG, and to a lesser degree corticosteroid-binding globulin. About 45% of blood Te is bound to high-affinity SHBG, 4% to corticosteroid-binding globulin, and the remaining 50% to lower-affinity high-capacity binding proteins, such as albumin. Approximately 1% to 2% remains free/unbound. DHT and anabolic steroids have greater affinity for SHBG than Te. Although of higher affinity, the 1000-fold lower molarity of plasma E 2 than Te in men limits any substantial depression by E 2 of Te’s binding to SHBG at physiologic pH and temperature. Low-affinity binding of Te (or E 2 ) to albumin may create a reservoir from which free Te (or E 2 ) arises rapidly due to an equilibrium dissociation half-time of about 0.2 seconds, compared with 3.8 seconds for SHBG. Significant one-pass dissociation is inferable in the liver, brain, and splanchnic circulation, but whether capillary-bed dissociation kinetics are organ selective is unknown. In one study, experimentally controlled free, bioavailable, and total Te concentrations equally predicted feedback onto GnRH/LH outflow in healthy men.
The traditional perspective is that protein binding mediates steroid-hormone retention and transportation in plasma, and restricts metabolism and inactivation. This view has been challenged by recent experimental data, since SHBG and albumin-bound steroids may also be biologically available for tissue-specific uptake. Although only free (unbound) hormones are believed to enter the cell by diffusion, SHBG may associate with presumptive high-affinity membrane-binding receptors, such as megalin, which may facilitate SHBG-bound Te’s entry into cells. Mutagenetic studies suggest that certain glycosylation sites in the C-terminus of SHBG are important for cell-surface (albeit not necessarily megalin) receptor recognition. Megalin-knockout mice exhibit apparent androgen resistance, inferable from impaired testicular descent into the scrotum. Genetic studies also show that the risk of type 2 diabetes is influenced by certain polymorphisms of the SHBG gene, which may not be related to SHBG-steroid binding. In addition, occupancy of membrane-bound SHBG by Te, DHT, or E 2 may activate intracellular Ca 2+ or cAMP, signaling purportedly via G-protein coupled and mitogen-kinase pathways.
SHBG and testicular androgen-binding protein (ABP) are both encoded by a single gene located on the short arm of chromosome 17. The proteins differ in their sites of production (SHBG by hepatocytes, brain, and placenta, and ABP by Sertoli cells and possibly brain), as well as oligosaccharide composition. SHBG secretion by hepatocytes is induced prominently by estrogens, thyroxine, and some anticonvulsant drugs, and repressed by insulin, growth hormone/IGF-I, and nonaromatizable androgens. The presumptive intratesticular role of testicular ABP is to maintain high intratubular total Te concentrations required for spermatogenesis. This notion is endorsed by the findings of increased germ-cell apoptosis and decreased fertility after transgenic overexpression of ABP in mouse testes.
Physiology of Hypothalamo-Pituitary-Testicular Network
- ◆
Hormone secretion in the male gonadal axis exists in both ultradian (pulsatile) and circadian.
- ◆
Biomathematical models of time-delayed nonlinear interactions among GnRH, LH, and Te have provided novel insights into male gonadal axis regulation.
- ◆
Te feedback is substantially mediated by aromatization to estradiol.
None of kisspeptin, GnRH, LH, or Te acts alone. Rather, all four signals are interlinked in a network or ensemble ( Fig. 12.6A ). Pulsatile GnRH secretion drives pulsatile LH secretion, which stimulates pulsatile Te production. Direct sampling of human spermatic-vein blood suggests that (1) Te feeds back onto both LH and FSH secretion within 30 to 45 minutes (LH) or longer (FSH); (2) LH, but not FSH, feeds forward onto Te secretion after a delay of 40 to 90 minutes; and (3) secreted Te distributes rapidly among free (aqueous), albumin-bound, and SHBG-bound compartments (see Fig. 12.6B ). These concepts form the basis for constructing biomathematical models of time-delayed, nonlinear interactions among GnRH, LH, and Te with ultradian (pulsatile) and circadian (24-hour rhythmic) features. Further, model advances require inclusion of locally modulated Te actions via ER subtypes, AR, and membrane receptors.
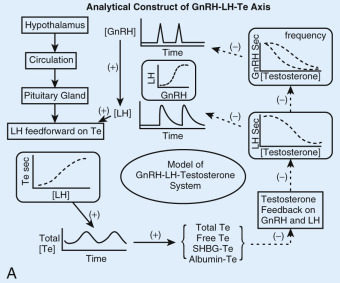
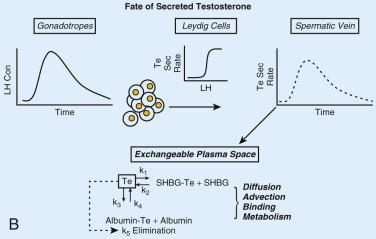
Both GnRH outflow and LH secretion are subject to feedback inhibition by Te and E 2 . Te feedback is substantially, but not completely, mediated by aromatization to E 2 systemically and/or within the hypothalamo-pituitary unit. Exogenous E 2 by acting on the pituitary reduces LH pulse amplitude without altering pulse frequency, whereas exogenous Te by acting on the hypothalamus and the pituitary gland decreases GnRH/LH pulse frequency and amplitude. Conversely, depletion of endogenous E 2 concentrations with an aromatase inhibitor or blockade of E 2 action with an ERα antagonist augments LH pulse size and number, therein mimicking the effects of nonsteroidal antiandrogens. In as much as E 2 depletion but not E 2 excess modulates GnRH/LH pulse frequency, endogenous estrogenic inhibition of the GnRH pulsing process (but not GnRH-driven LH pulse size) may be already maximal in normal men.
The characteristics of Te delivery may modulate inhibition of LH, since greater suppression is enforced by continuous than pulsatile Te infusions. This outcome supports the male hormonal-contraceptive approach of sustained Te administration to suppress LH and thereby intragonadal Te concentrations. Endogenous DHT plays a lesser role in feedback onto GnRH and LH, given that patients with 5α-reductase type II deficiency exhibit minimally elevated LH concentrations. Administration of a potent combined type I and II 5α-reductase inhibitor, dutasteride, in healthy young men, reduced systemic DHT concentrations by 95% and stimulated basal (constitutive) LH (and Te) secretion. This mechanism could explain the rise in all three mean, nadir, and peak LH concentrations in patients with 5α-reductase deficiency.
FSH concentrations are also regulated by negative feedback. Aromatization of Te to E 2 appears to be critical because (1) combined deprivation of Te and E 2 and isolated depletion of E 2 elevate FSH concentrations to comparable degrees ; (2) physiologic replacement of either Te or E 2 in hypogonadal men normalizes FSH concentrations; and (3) an aromatase inhibitor fully reverses suppression of FSH by Te. Although nonaromatizable androgens can also suppress FSH and LH secretion, the study paradigms have been pharmacologic rather than physiologic.
Nonsteroidal testicular products, such as inhibin B secreted by Sertoli cells, may be involved in feedback regulation in animals. In humans, inhibin B concentrations exhibit a multimodal lifetime pattern. Values in umbilical cord blood are comparable to peripheral-venous levels in men, increase further in infancy, decline during subsequent childhood, rise in puberty, and fall by 25% by the eighth decade of life. The changes are paradoxically concordant (birth through puberty) and reciprocal (adulthood) with FSH, LH, and Te secretion, indicating that other factors modulate both FSH and inhibin B. Albeit supported by corollary data, the role of inhibin B in restraining FSH production in vivo has been inferable only in the adult male rodent and monkey. To date, no clinical studies have infused or neutralized inhibin B in men, and no human mutations of inhibin B or its receptor have been described. The same qualifications apply to the roles of activin A, a homodimer that stimulates FSH-β gene expression, and follistatin, a linear peptide that binds to and antagonizes feedforward by activin. Inhibins and activins signal via transforming growth-factor (TGF) β receptors and β-glycan coreceptors. TGF receptors also strongly regulate cell proliferation, apoptosis, tumorigenesis, and hematopoiesis. Contraceptive applications of safe and effective mimetics of inhibin B and antagonists of activin A remain to be exploited.
Testis
- ◆
The testis is encapsulated in the tunica albuginea and consists of 250 to 300 lobules.
- ◆
Each lobule contains one to four segments of seminiferous tubules.
- ◆
Male-sex determination and testicular morphogenesis are orchestrated by sex-determining region of the Y chromosome (SRY).
The adult testes are paired ovoid structures suspended in the scrotum by the spermatic cord. Prepubertal testis volume is less than 7 mL, and postpubertal greater than 12 mL. Each is encapsulated by the fibrous tunica albuginea (“white cloak”), which is invaginated posteriorly to form the mediastinum testis perforated by neurovascular and lymphatic vessels and efferent ductules. Efferent ductules form from the rete (“net-like”) testis, which represents the convergence of all seminiferous tubules. Fibrous septa project from the tunica albuginea into the testis, dividing it into 250 to 300 lobules. Each lobule contains one to four segments of seminiferous tubules. The latter develop at the time of puberty through canalization of the seminiferous cords. Peritubular-myoid and Sertoli-cell interactions mediate formation of seminiferous cords. Spermatogenic tubules are lined internally by Sertoli cells, which support the proliferation and differentiation of spermatogonial cells by secreting trophic and prosurvival factors, like stem-cell factor ( c-kit ligand), which nourish and inhibit apoptosis of germ cells. Sertoli cells protect spermatogonia by maintaining a unique tubular microenvironment through tight junctional complexes that constitute the blood-testis barrier. Conversely, spermatogonia and peritubular myoid cells both signal to Sertoli cells. Sertoli cells abut a basement membrane, which is lined externally by peritubular myoid cells. Myoid cells are surrounded by an interstitium containing Leydig cells, lymphatics, venules, and nerve fibers. Peritubular myoid, Leydig, and Sertoli cells together regulate spermatogenesis.
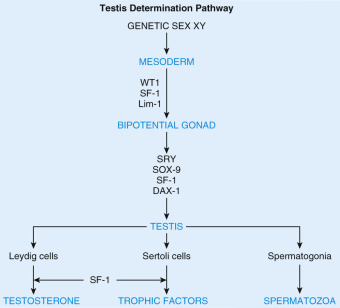
Testes develop intraabdominally and descend into the scrotum perinatally. Insulin-like 3 induction of gubernacular development is essential for transinguinal migration. Male-sex determination and testicular morphogenesis are orchestrated by the SRY, which is both necessary and sufficient for testis formation ( Fig. 12.7 ). SRY in turn activates a family of homeobox-related genes, in particular Lim1 and SOX9, as well as steroidogenic-factor 1 (SF1). SF1 is a member of the NR5A subfamily of nuclear receptors required for testis, adrenal, and gonadotrope development. Mutations in SRY, SOX9, and SF-1 or its repressor, the dosage-sensitive X-linked sex-reversal (DAX1) gene, are responsible for the majority of known causes of aberrant sexual development.
Accessory Organs
- ◆
Epididymis (upper segment), vas deferens (middle), and ejaculatory duct and seminal vesicles (lower) develop from wolffian (mesonephric) ducts under the influence of Te.
- ◆
In contrast, the prostate arises from the urogenital sinus under the influence of DHT.
Under the influence of Te, embryonic wolffian (mesonephric) ducts develop into the internal male reproductive tract—namely, the epididymis (upper segment), vas deferens (middle), and ejaculatory duct and seminal vesicles (lower). By the fourth week of embryonic life, the ejaculatory ducts arch anteriorly to join the ventral portion of the cloaca. The cloaca subsequently differentiates into the urogenital sinus. DHT is required for development of the prostate gland, urethra, scrotum, and penis from the urogenital sinus.
Vas Deferens
Vasa deferentia transport sperm by means of muscular layers that propel spermatozoa during ejaculation. In the absence of ejaculation, spermatozoa stored in each vas deferens dribble through the terminal dilated ampulla into the ejaculatory duct, where they are washed away during micturition. The distal urethral meatus discharges urine or semen from the body.
Epididymis
The epididymis is formed from a single duct through which all spermatozoa pass, mature, gain fertilizing capability, and become motile before entering the vas deferens and ejaculatory duct. The human epididymis is 4 to 5 cm long and consists of the caput, corpus, and cauda. The cauda in man appears to differ from that in other species by way of more rapid transit of spermatozoa (2 to 4 days) and a less differentiated initial segment. Peptides and nonproteinaceous molecules are secreted into the epididymal lumen by principal cells. Although little is known in any species about precise regulation of luminal fluids that modulate sperm maturation and motility, microarray profiling of human epididymal tissue has demonstrated highly regionalized gene expression with maximal complexity in the caput and least in the corpus. The maturation process is complete by the time spermatozoa enter the proximal (caudal) epididymis and are able to hyperactivate, bind the zona pellucida of the oocyte, and undergo the acrosome reaction.
The micromilieu of spermatozoa stored in the cauda epididymidis remains poorly understood. Ductal mucosa must play a critical role in controlling solute concentrations and degree of sperm hydration. Muscular cells exist only in the cauda epididymides and ductus deferens, and hence repeated ejaculation increases the rate of sperm transport from these regions only.
Epididymal growth, development, and function are dependent on androgens, estrogens, and other nonsteroidal luminal factors. Luminal androgen concentrations exceed those in peripheral blood, and for this reason systemic androgen exposure may not be critical for epididymal development and function. Amplification of luminal Te effects occurs by local synthesis of DHT via gonadal 5α-reductase type II.
Seminal Vesicles
The seminal vesicles secrete the majority of fluid found in the ejaculate. Secretion includes fructose, prostaglandin, semenogelin I (responsible for coagulation of the seminal fluid), reactive-oxygen scavengers (superoxide dismutase, catalase), and immunoglobulin G receptor III. During embryogenesis, androgens transform simple saccular anlagen into active secretory epithelium by branching morphogenesis, yielding a highly folded internal structure with an expanded surface area. The precise molecular mechanisms are not yet defined.
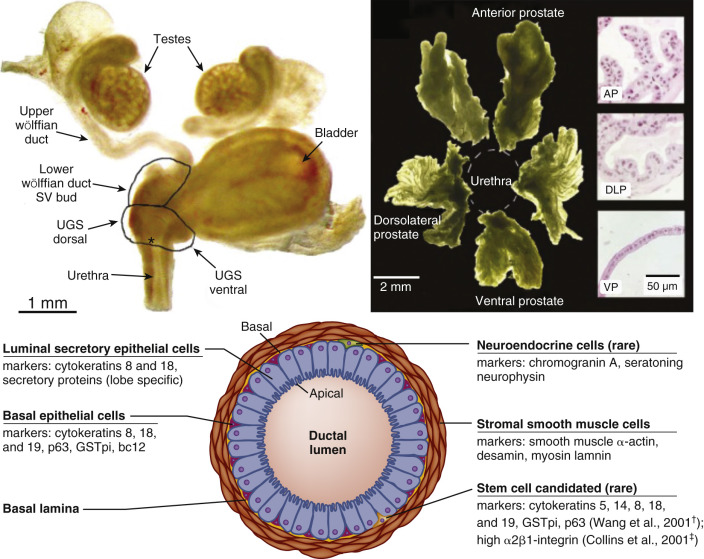
Prostate Gland
The prostate is the largest accessory gland, but unlike the epididymis and seminal vesicles, arises from the urogenital sinus rather than mesonephric ducts and requires DHT action ( Fig. 12.8 ). Prostatic fluids, which comprise about one-third of the volume of the ejaculate, contain high concentrations of zinc, citric acid, choline, and proteins such as acid phosphatase, seminin, plasminogen activator, and prostate specific antigen (PSA). PSA is an important blood marker of prostate size and proliferation. PSA concentrations are elevated in benign prostate hypertrophy, inflammation, and prostatic cancer. The prostate gland develops from distinct sets of tubules that initially form discrete lobes, which become confluent by adulthood. Thus the adult prostate gland is generally divided into peripheral, central, and transitional zones according to morphologic and functional properties rather than lobular structure. The peripheral zone is subcapsular and surrounds the distal urethra, the central zone encircles the ejaculatory ducts, and the transition zone envelops the proximal urethra. The peripheral and central zones comprise respectively 70% and 25% of total prostate-gland volume in young men, accounting for equivalent proportions of prostate cancer in later life. In contrast, prostate cancer rarely arises from the transition zone (5% of volume), where benign prostate hypertrophy usually develops.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree