Abstract
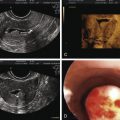
A transvaginal ultrasound (TVS) is an efficient imaging tool in the “one-stop” investigation of subfertility. A comprehensive evaluation of the structure of ovarian, uterine, and tubal patency can provide immediate information in the evaluation and thus counseling of the patient. Improvements in ultrasound technology allow for a detailed assessment of the ovary, and an estimation of ovarian functional status. The use of three-dimensional volume sets allows for the acquisition of hundreds of images of the uterine contour, and thus detection of the presence of müllerian anomalies, intracavitary filling defects, and myometrial pathology. A TVS is essential in the provision of early pregnancy care.
Key words
Ultrasound investigation for subfertility, PALM-COEIN, müllerian anomalies, deeply invasive endometriosis, miscarriage, pregnancy of unknown location
A single-visit, “one-stop” or “pivotal,” ultrasound-based approach in the investigation of subfertility has gained widespread acceptance ( Table 35.1 ). Two-dimensional (2D) ultrasound is the primary tool for the initial investigation of the pelvis and provides a diagnostically accurate, minimally invasive, and cost-effective assessment tool. Technological improvements in high-resolution transvaginal probes, and the use of color Doppler imaging, have allowed for the meticulous assessment of detail within these anatomic structures—called sonomicroscopy —and not only enhanced diagnostic capabilities but elucidated critical data into the functional status of these organs (e.g., assessment of ovarian reserve [OR] with an antral follicle count [AFC], or studying the ovarian morphology in the diagnostic evaluation of the patient with suspected polycystic ovary syndrome [PCOS]). Three- and four-dimensional (3D/4D) volume ultrasound imaging can provide images of the pelvis comparable in quality and orientation to those of magnetic resonance imaging (MRI) and computed tomography (CT), but without the radiation and at relatively lower cost. The relative ease and efficiency of the acquisition of 3D volume ultrasound sets allow for the storage of entire volumes of imaging. This enables the offline examination and manipulation of additional images obtained within the volume set, with the possibility of creating hundreds of new images, and perhaps future consultation with expert sonographers (e.g., müllerian anomalies). Further, the dynamic nature of ultrasound imaging of the pelvis has the added advantage of enhancing a detailed physical exam, allowing for the additional inquiry of sites that might elicit the patient’s symptoms such as pain, and detect the presence of adhesions, and thus correlate those symptoms with specific anatomic findings.
| High resolution transvaginal ultrasound approach preferred |
| Proliferative phase (~CD 4–9) |
| Uterus and Uterine Cavity |
|
| Endometrium |
|
| Ovarian |
|
| Tubal Patency |
|
| Cul-de-sac |
|
Ultrasound Examination Technique
- ◆
A dynamic ultrasound exam of the pelvis allows for a detailed assessment of pelvic anatomy in addition to elucidating potential sources of pelvic pain and adhesions.
- ◆
Measurement of the uterus and ovaries may be important diagnostic tools in the evaluation of the pubertal patient with an endocrinopathy.
- ◆
Sonohysterography (SHG), or saline infusion sonography, is highly likely to aid in the detection of intracavitary pathology.
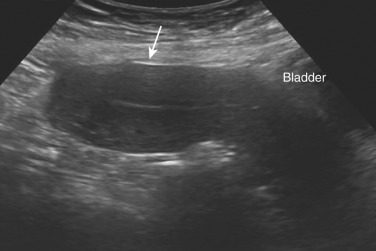
In most women, a transvaginal sonographic (TVS) approach in the early proliferative phase of the menstrual cycle (~cycle day 4 to 9), when the patient is not actively bleeding and prior to ovulation, is preferred. A transabdominal scan (TAS) may be required for imaging of pelvic structures beyond the focal length of the probe, and/or out of the pelvis, such as in the case of an enlarged myomatous uterus, a uterus adherent to the anterior abdominal wall ( Fig. 35.1 ), or an ovary adherent out of the pelvis. When a TVS is considered inappropriate (e.g., patients who are virginal, vaginismus, or secondary to vaginal stenosis), or if the TAS is inconclusive, a transrectal ultrasound exam should be considered. The bladder is often emptied prior to the initiation of a TVS, but it may be necessary to fill the bladder a small amount to displace the small bowel from the field of view to enhance the quality of the imaging. Image quality during TAS may be hampered by adiposity, scar tissue, bowel gas, or uterine retroversion.
Examination by TVS commences with a dynamic 2D scan of the vagina, bladder, and cervix. The position of the uterus is noted and measurements are taken. An entire overview of the uterus is obtained with the scanned proceeding in the sagittal plane from cornu to cornu, and in the (oblique) transverse plane from cervix to fundus. The uterine corpus view is then magnified as large as possible, focusing on the area of interest. Gentle pressure applied by the examiner’s free hand on the abdomen and simultaneously applied to the vaginal probe may be used dynamically to assess uterine, cervical, and ovarian mobility, and their possible pathologies relative to the static pelvic wall, and/or adherence to the large and small bowel. Difficulties in imaging of the detail of the uterine anatomy may arise from variations of the uterine position (particularly when axial), or with uterine rotation secondary to adhesions. This dynamic exam allows for the identification of the possible presence of adhesions, such as uterine fundal attachment to the anterior abdominal wall, or adhesions of the uterus to the colon such as in cul-de-sac obliteration in endometriosis. The dynamic ultrasound guided exam may also serve to pinpoint the potential causes of pain and to screen for site-specific tenderness.
Ultrasonographic measurements of pelvic organs show a high level of agreement with those measured with geometric calipers shortly after surgical excision. In 28 women planning total abdominal hysterectomy, Saxton et al. compared transabdominal ultrasound (TAU) pelvic organ measurements obtained in the perioperative period to absolute dimensions of the excised pelvic organs and found no significant difference for the two methods for uterine cross-sectional area ( r = −0.26), endometrial thickness (EMT; r = 0.29 mm), and right ovary ( r = 0.14). Measurements for the left ovary were significantly different when the left ovary was larger (>10 cm 3 ; r = 0.55), a finding attributed to the potential confounders of measuring the left ovarian measurement on TAU (bowel gas and distortion by adjacent sigmoid colon anatomy). Further evidence to support the ability of ultrasound to mirror clinical data includes a nearly perfect correlation between the number of follicles observed at ultrasound (US) and the histologic number documented in the polycystic ovary (PCO) morphology, and the degree of ovarian stromal hyperplasia (a marker for androgen excess).
Evaluation of the Uterus
In clinical practice the uterine corpus is measured in three dimensions, the symmetry of the myometrial walls is estimated, and the overall echogenicity of the myometrium is reported as homogeneous or heterogeneous ( Table e35.1 ) (see Chapter 26 ). If a myometrial lesion is observed, it is described as well-defined or ill-defined, the number (or an estimated number if there are more than four lesions) is reported, as well as location and maximal diameter of the clinically relevant lesion(s) ( Fig. 35.2 ). The presence of shadowing, myometrial cysts, hyperechogenic islands, and/or subendometrial echogenic lines and buds is reported. The anterior and posterior myometrial walls are measured from the external uterine serosa to the external endometrial contour and should include the junctional zone (JZ) but exclude the endometrium. The total length of the cervical canal is measured after identifying the external os of the cervix. The two primary constituents of the uterus—the myometrium and the endometrium—are present at an interface that is easily identifiable on ultrasound. The lower end of the endometrium delineates the internal os of the cervix, which provides the landmark for individual measurements of the cervix and the uterine corpus.

| Feature to Be Described | Description/Term |
|---|---|
| Uterine corpus | Length, anteroposterior diameter, transverse diameter (cm) |
| Myometrial walls | Symmetrical/asymmetrical |
| Overall echogenicity | Homogeneous/heterogeneous |
| Myometrial lesions | Well-defined/ill-defined |
| Number | Number (1, 2, 3, or estimated in case of >4 lesions) |
| Location | Location of the largest/clinically relevant lesion(s): anterior, posterior, fundal, right lateral or left lateral, global |
| Site | Site (for well-defined lesions) of the largest/clinically relevant lesion(s): FIGO classification 1–7 |
| Size | Maximum diameter of the largest/clinically relevant lesion(s) |
| Shadowing | — |
| Edge shadows | Present/absent |
| Internal shadows | Present/absent |
| Fan-shaped shaped shadowing | Present/absent |
| Cysts | Present/absent |
| Hyperechogenic islands | Present/absent |
| Subendometrial echogenic lines and buds | Present/absent |
| Junctional zone | Regular/poorly defined |
| Vascularity of myometrium | — |
| Overall vessel pattern (in the whole uterus) | Uniform/nonuniform |
| Amount of color (in a lesion): color score | (1) No color, (2) minimal color, (3) moderate color, (4) abundant color |
The JZ (also referred to as the inner myometrium, archimyometrium, or stratum subvasculare) is visible as a hypoechogenic subendometrial halo and is of müllerian origin ( Fig. 35.3 ). It lies between the echogenic basal layer of the endometrium and the underlying myometrium. The JZ is confirmed as a distinct structure not only because of its embryonic origin but also in terms of its specialized functions. The JZ is composed of longitudinal and circular closely packed smooth-muscle fibers that are endowed with estrogen receptors (ER) and progesterone receptors (PR) and exhibit a cyclical pattern which parallels that of the endometrium. A systematic morphometric and immunohistochemical analysis of the JZ reveals the JZ myometrium consists of normal myocytes, albeit with a greater nuclear-cytoplasmic ratio, compared with the smooth muscle cells of the outer myometrial zone. This cellular architecture suggests the overall water content is relatively lower in the JZ, which in turn alters its acoustic impedance and accounts for its hypoechogenic, “halo,” appearance on TVS. The JZ also undergoes cyclical changes in thickness which, again, mimic, but are less pronounced that those in the endometrium. The JZ is described as regular, or poorly defined, and if it is irregular, interrupted, not visible or assessable. The outer, non-müllerian layers of the myometrium have constant concentrations of ER and PR throughout the menstrual cycle.
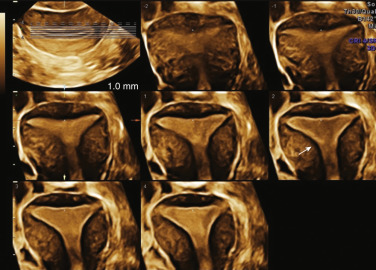
Advances in real-time TVS technology have demonstrated that propagated myometrial contractions in the nonpregnant uterus originate from the JZ. The orientation, amplitude, and frequency of these JZ contraction waves are dependent on the phase of the menstrual cycle. In the follicular and periovulatory phases, cervicofundal subendometrial contractions are observed, the amplitude and frequency of which increase notably toward ovulation. Short, asymmetrical myometrial waves are present during the luteal phase, and during menstruation, propagated fundocervical subendometrial contractions have been observed. These subendometrial cervicofundal contraction waves have been demonstrated to facilitate rapid sperm transport through the female genital tract. It has been postulated that the asymmetrical myometrial peristalsis during the luteal phase serves to maintain the developing blastocyst within the uterine fundus. A low frequency of JZ contractility prior to embryo transfer (ET) is associated with higher implantation and clinical pregnancy rates. These observations indicate that while the JZ myometrium is morphologically similar to the outer myometrium, it is functionally more akin to the endometrium in both dynamic changes in response to the rise and fall of ovarian steroidal hormones.
On sonographic cross-section through the uterus, the arcuate arterial and venous vessels can be seen in close proximity to the outer myometrial border. When it is clinically relevant to evaluate vascularity (e.g., myometrial masses), the overall vessel pattern within myometrium of the entire uterus is reported as uniform or nonuniform. The amount of color within a lesion is reported using the color score (1 = no color; 2 = minimal color; 3 = moderate color; 4 = abundant color) as defined by the Morphological Uterine Sonographic Assessment (MUSA) group consensus statement on terms and definitions to describe the myometrium. Subjective color scoring (0 to 4) may be applied to the ovarian masses, myometrial masses, and in the assessment of endometrium (see Fig. 35.8 ).
In premenopausal adult women, total uterine length including the cervix ranges from 6.5 to 10 cm, with uterine size showing a progressive increase in length and width, correlated with parity ( Table e35.2 ). In a group of 155 premenopausal women from 16 to 52 years (mean age 32.4), Merz et al. reported a significant difference in uterine length in nulliparas (7.3 cm ± 0.8 [SD]), primiparas (8.3 cm ± 0.8 [SD]), and the group of multiparas (9.2 cm ± 0.8 [SD]). Significant differences were noted in those who had delivered once and those who had delivered twice or more. In the postmenopausal group ( n = 108) not exposed to exogenous estrogen (mean age 62 years), patients were stratified to those who were menopausal 5 years or less and those with menopause more than 5 years. Between these two groups there was a statistically significant difference in size (6.7 cm ± 0.7 [SD] vs. 5.6 cm ± 0.9 [SD]) with duration since menopause.
| Structure (cm) ± (SD) | Para 0 ( n = 52) | Para 1 ( n = 50) | Para ≥ 2 ( n = 53) | Menopause ≤ 5 years ( n = 44) | Menopause > 5 years ( n = 64) |
|---|---|---|---|---|---|
| Uterine length | 7.3 ± 0.8 | 8.3 ± 0.8 | 9.3 ± 0.8 | 6.7 ± 0.7 | 5.6 ± 0.9 |
| Corpus length | 4.4 ± 0.6 | 4.9 ± 0.6 | 5.6 ± 0.9 | 3.8 ± 0.5 | 3.3 ± 0.6 |
| Corpus width | 4.0 ± 0.6 | 4.6 ± 0.5 | 5.1 ± 0.5 | 3.6 ± 0.5 | 3.1 ± 0.5 |
| Corpus height | 3.2 ± 0.5 | 3.9 ± 0.5 | 4.3 ± 0.6 | 3.1 ± 0.4 | 2.5 ± 0.4 |
| Cervix length | 2.9 ± 0.5 | 3.4 ± 0.5 | 3.7 ± 0.6 | 2.9 ± 0.4 | 2.4 ± 0.5 |
| Cervix width | 2.9 ± 0.5 | 3.3 ± 0.5 | 3.4 ± 0.5 | 2.7 ± 0.5 | 2.3 ± 0.4 |
| Cervix height | 2.6 ± 0.4 | 2.7 ± 0.4 | 3.0 ± 0.6 | 2.4 ± 0.4 | 2.1 ± 0.4 |
| Corpus-cervix ratio | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.5 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.2 |
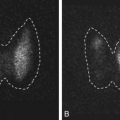
In the first month of life, uterine volume declines and remains low until approximately age 7 to 9. This initial decline has been confirmed by anatomical studies and is a consequence of the withdrawal of maternal estrogens after birth. Haber and Mayer performed TAU on 178 girls between the ages of 1 day and ![]() months, and calculated uterine and ovarian volumes (OVs) using the formula for a prolate ellipsoid, demonstrating a decline in uterine size in the first month of life, 3.4 ± 1.2 mL versus 0.9 ± 0.2 mL at 3 months, with the most marked change noted in the first 6 weeks of life. OV, calculated as the mean of both ovaries, remained unchanged in the first 2 years of life (0.5 ± 0.3 mL). Uterine volume remained constant in their study population until the onset of puberty, with uterine volume increasing ahead of OV, and may be one of the first markers of puberty. Hagen et al. observed 121 healthy girls aged 9.8 to 14.7 years and documented uterine volume by 3D US and MRI, and correlated uterine volume and EMT with pubertal stages. There was a strong correlation between uterine and endometrial volumes calculated with ellipsoid MRI and 3D US. Their data from late prepubertal girls (Tanner stage B1, median age 10.9 years; median volume 4.1 cm 3 ) provided further evidence that uterine and endometrial growth precedes development of breast tissue. It has been postulated that prepubertal uterine growth may be caused by low concentrations of estradiol (E 2 ) originating from the small number of large follicles present, even in prepubertal girls and/or E 2 aromatized from adrenal androgens. Indeed, data such as these may provide a valuable clinical resource to help distinguish between precocious puberty and isolated precocious thelarche in girls.
months, and calculated uterine and ovarian volumes (OVs) using the formula for a prolate ellipsoid, demonstrating a decline in uterine size in the first month of life, 3.4 ± 1.2 mL versus 0.9 ± 0.2 mL at 3 months, with the most marked change noted in the first 6 weeks of life. OV, calculated as the mean of both ovaries, remained unchanged in the first 2 years of life (0.5 ± 0.3 mL). Uterine volume remained constant in their study population until the onset of puberty, with uterine volume increasing ahead of OV, and may be one of the first markers of puberty. Hagen et al. observed 121 healthy girls aged 9.8 to 14.7 years and documented uterine volume by 3D US and MRI, and correlated uterine volume and EMT with pubertal stages. There was a strong correlation between uterine and endometrial volumes calculated with ellipsoid MRI and 3D US. Their data from late prepubertal girls (Tanner stage B1, median age 10.9 years; median volume 4.1 cm 3 ) provided further evidence that uterine and endometrial growth precedes development of breast tissue. It has been postulated that prepubertal uterine growth may be caused by low concentrations of estradiol (E 2 ) originating from the small number of large follicles present, even in prepubertal girls and/or E 2 aromatized from adrenal androgens. Indeed, data such as these may provide a valuable clinical resource to help distinguish between precocious puberty and isolated precocious thelarche in girls.
The uterus grows more rapidly in response to estrogen at the time of puberty. In a prospective trial examining 139 girls between the ages of 1 and 13 years of age with radiological bone age measurements, uterine volume was calculated following TAU values based on the formula for a prolate ellipse. Uterine measurements and volume were markedly smaller in prethelarche girls at 1.8 ± 1.2 cm 3 , as compared with 8.1 ± 6.6 cm 3 after thelarche. In a large prospective trial conducted on 380 school girls 6 to 18 years of age, Holm and colleagues observed no age-related differences in uterine and ovarian size in 44 prepubertal girls, whereas a direct correlation with Tanner stages was observed in 163 prepubertal girls. A further enlargement of the uterus was observed between Tanner stage 3 and adult girls (mean age 19).
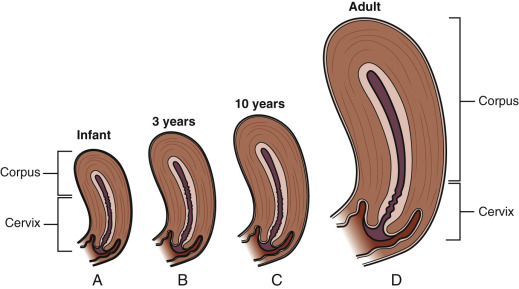
An awareness of the normal size and proportions of the cervix and uterus is obligatory when imaging the newborn and prepubertal girl. Prenatally there is linear growth of the uterus until the seventh prenatal month, when there is an upsurge in growth of the uterine corpus, followed by a drastic postnatal regression within the first 6 weeks of postnatal life. Postnatal regression is attributed to hypoplasia and atrophy of the myometrium, with a concomitant natal reduction in uterine blood flow ( Fig. 35.4 ). In infancy, the cervix is much longer in proportion to the corpus, is associated with a lack of anteflexion, with a cervical axis in line with the longitudinal axis of the vagina. The uterus has a tubular shape with a fundus/cervix ratio of around 1. In infancy, the corpus is described as no larger than a shelled almond, is stationed higher within the pelvis, and the corpus is approximately one-half the diameter of the cervix. The endometrium is in the resting phase and relatively thin.
There are endocrine conditions in which the use of pelvic ultrasound may serve as a noninvasive and efficient diagnostic tool in the assessment of the patient (see Chapter 17 ). Although the gonadotropin-releasing hormone (GnRH) stimulation test is considered the “gold standard” for differentiating central precocious puberty (CPP) from GnRH-independent forms of precocious puberty, pelvic ultrasound is considered a noninvasive, rapid, and reliable tool for imaging the internal genitalia of girls. Several investigators have demonstrated the usefulness of TAU to help differentiate children with CPP from those with premature thelarche (PT). In a study to compare pelvic ultrasound measurements in normal girls with various forms of sexual precocity (PT, premature pubarche, and CPP), Badouraki et al. ( Table e35.3 ) reported that in girls aged 0 to 6 years, patients with CPP have significantly increased uterine volume, OV, and fundus/cervical (F/C) ratio as compared with controls, and significantly increased uterine length, OV, and F/C ratio compared with girls with PT. All ultrasound measurements were significantly increased in patients with CPP compared with girls with premature pubarche. With regard to age group greater than 6 to 8 years, patients with CPP showed significantly high values for all the pelvic ultrasound measurements compared with controls and girls with PT, and all ultrasound parameters were significantly increased in girls with CPP compared with controls in the age group 8 to 10 years. In assessing receiver operator curves (ROC), OV seemed to be the best parameter for identifying patients with CPP in the age groups 0 to 6 and greater than 6 to 8 years and to be equivalent value with uterine volume for identifying patients with CPP ages 8 to 10 years. Further, regarding the adequacy of treatment for CPP with gonadotropin-releasing hormone analogues (GnRHa), de Vries et al. demonstrated that following 3 months of treatment there was a significant decrease in most uterine and ovarian parameters.
| Parameter | Controls | Premature Thelarche | Premature Pubarche | Central Precocious Puberty |
|---|---|---|---|---|
| Age 0–6 Years | ||||
| Uterine length (cm) | 2.81 ± 0.43 | 2.89 ± 0.43 | 2.85 ± 0.75 | 4.44 ± 1.14 * † |
| Uterine volume (cm 3 ) | 1.91 ± 0.47 | 2.76 ± 1.71 | 1.9 ± 1.1 | 4.78 ± 2.6 † ‡ |
| Ovarian volume (cm 3 ) | 1.26 ± 0.54 | 1.15 ± 0.71 | 0.97 ± 0.45 | 2.62 ± 1 * † ‡ |
| F/C ratio | 0.93 ± 0.09 | 0.91 ± 0.1 | 0.93 ± 0.1 | 1.33 ± 0.32 † ‡ |
| Age > 6–8 Years | ||||
| Uterine length (cm) | 3.27 ± 0.35 | 3.3 ± 0.41 | 4.12 ± 0.47 | 4.16 ± 0.73 * ‡ |
| Uterine volume (cm 3 ) | 2.58 ± 0.88 | 2.72 ± 1.08 | 3.61 ± 1.93 | 4.82 ± 1.59 * ‡ |
| Ovarian volume (cm 3 ) | 1.71 ± 0.67 | 1.83 ± 0.92 | 2.06 ± 0.67 | 2.77 ± 0.97 * ‡ |
| F/C ratio | 1.02 ± 0.08 | 1 ± 0.06 | 1.08 ± 0.3 | 1.21 ± 0.18 * ‡ |
* Statistically significant compared with patients with premature thelarche.
† Statistically significant compared with patients with premature pubarche.
Alternatively, there are endocrine disorders associated with decreased uterine and ovarian size. Doerr and colleagues report on a series of 75 women affected by Turner syndrome (TS) (45,X 78.6%; 45,X/46,XX 5.4%; 45,X/46,X,i[Xq] 8%; 45,X/46,XY 8%). Of 50 women with a karyotype (45,X) who had received estrogen replacement and growth hormone (GH) therapy to induce puberty, 42/50 (84%) had a normal size uterus, whereas it was smaller (<5 cm) in the remaining eight women (16%). In this study, uterine size might have been affected by the age at which estrogen therapy was initiated, but showed no correlation with other factors, notably the final height. In calculating their data in standard deviation scores (SDS), only women with 45,X/46,XX karyotype had normal median uterine length and volume, whereas 26% of the TS women with karyotype 45,X had a uterine length less than −2 SDS and a volume of less than −2 SDS. Despite adequate estrogen replacement, incomplete breast development (Tanner stage B3) was found in women with 45,X karyotype ( n = 11, 18.6%). Since onset and course of breast development and uterine growth are closely correlated, these results led these authors to hypothesize a disturbance affecting uterine and mammary structural anomalies may exist in some patients with TS 45,X. The impact of GH on uterine size remains a debate, with Sampaolo and colleagues and Doerr et al. showing a positive effect, whereas Snajderova and colleagues found none. Of note, not all authors have analyzed their data based on karyotype subpopulations. Uterine and ovarian growth may be similarly affected in other endocrine patient populations with persistent low levels of estrogen. Patients with functional hypothalamic amenorrhea, anovulation associated with anorexia nervosa, and hypopituitarism causing hypogonadotropic hypogonadism all have documented smaller uterine size.
Diethylstilbestrol (DES) is a nonsteroidal estrogen that was used to prevent pregnancy loss and complications but was subsequently found to be ineffective. Starting in the late 1930s through the early 1970s, DES was given to at least 2 million pregnant women in the United States alone. In the late 1960s, an unusual cluster of cases of clear-cell adenocarcinoma of the vagina and cervix in adolescent girls was observed at one hospital. The clinicians involved, working with the mothers of these women, discovered a strong association between an otherwise excessively rare tumor with the in utero exposure to DES. There are well-documented consequences of prenatal DES exposure in adult women that include structural anomalies of the reproductive tract, menstrual irregularity, infertility, pregnancy loss, preterm delivery, loss of second-trimester pregnancy, ectopic pregnancy (EP), preeclampsia, stillbirth, early menopause, grade 2 or higher cervical intraepithelial neoplasia, breast cancer at 40 years of age or older, and elevated risk of clear cell cancers of the vagina. Studies of reproductive tract tissues of mice indicate that the adverse effects of developmental DES exposure are due to epigenetic mechanisms involving persistent changes in gene expression.
Structural changes of the uterine cavity are seen in up to 70% of women exposed to DES in utero, and include T-shaped uterine cavity, a hypoplastic uterine cavity ( Fig. 35.5 ) with evidence of constriction bands, uterine filling defects, synechiae, and diverticuli. In a study comparing 18 DES-exposed women with 20 age-matched controls, Viscomi and colleagues utilized TAU to assess uterine volume. These authors reported a significant reduction in uterine size in the DES-exposed adult females, with a mean uterine length of 6.8 ± 0.4 cm as compared with 8.1 ± 0.8 cm in the control group women. Calculated uterine volume was 49.4 in the DES-exposed women and 90 cm 3 in the control group population.
Evaluation of the Endometrium
The endometrium is hormonally sensitive and changes in thickness, volume, and echogenicity during the menstrual cycle, and in response to hormonal treatment (see Chapter 9 ). In general, the endometrium is easily visualized; however, difficulties in assessing the full endometrial bilayer may arise from variations in uterine position (particularly when axial) or with uterine rotation (secondary to adhesions or endometriosis). These problems may be overcome by filling the bladder, or performing a dynamic scan by pressing on the abdomen with the free hand. The International Endometrial Tumor Analysis (IETA) group has released a consensus statement on terms, definitions, and measurements to describe the sonographic features of the endometrium and uterine cavity at grayscale sonography, color flow imaging, and SHG. The uterine cavity can also be divided into an “unenhanced” or “enhanced” ultrasound examination of the uterine cavity, depending on whether the operator decided to instill fluid ( Table 35.2 ).
| Unenhanced Ultrasound | Descriptor |
|---|---|
| Endometrial thickness | Measurement in _____ mm/not measureable |
| Endometrial echogenicity and pattern | Uniform: hyperechoic/hypoechoic/isoechoic |
| Nonuniform: Homogeneous with or without irregular cystic areas, or Heterogeneous without cystic areas, or with regular or irregular cystic areas | |
| Endometrial midline | Linear/nonlinear/irregular/not defined |
| “Bright edge” | No/Yes |
| Endo-myometrial junction | Regular/irregular/interrupted/not defined |
| Synechiae | No/Yes |
| Intracavitary fluid | No or Yes with echogenicity of fluid described as anechoic/low level echogenicity/ground glass/“mixed” echogenicity |
| Color Doppler assessment | Color Score within the endometrium: 1 (no flow); 2 (minimal flow); 3 (moderate flow); 4 (abundant flow) |
| Vascular pattern: no vessels/single “dominant” vessel with or without branching/multiple “dominant” vessels focal origin or multifocal in origin/scattered vessels/circular flow |
Unenhanced Ultrasound of the Uterine Cavity
Quantitative Assessment of Endometrial Thickness, Intrauterine Lesions, and Intracavitary Fluid
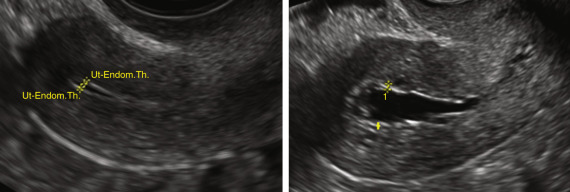
The EMT is the maximum measurement in the sagittal plane and includes both endometrial layers (double EMT). The endometrium should be measured where it appears the thickest perpendicular to the endometrial midline, with the calipers placed at the level of the two opposite endometrial-myometrial interfaces in an appropriately magnified image. The total double-layer thickness is reported in millimeters, rounded off to one decimal point. When intracavitary fluid is present, the thicknesses of both single layers are measured and reported separately ( Fig. 35.6 ), and the amount of intracavitary fluid is defined by its largest measurement in the sagittal plane. If the endometrium is thickened asymmetrically, the largest anterior and posterior EMTs should be measured and reported separately. When the endometrium cannot be seen clearly in its entirety, it should be reported as “nonmeasurable,” and no attempt should be made to measure it. The proportion of cases in which the endometrium cannot be measured may be as high as 10% in perimenopausal women. It is likely much higher in postmenopausal women, although this has not been adequately studied. When intracavitary pathology is present, other than an intracavitary myoma, the total EMT including the lesion is measured and recorded. If an intracavitary myoma is present, it is not included in the EMT measurement, and the three perpendicular measurements are obtained and recorded. In myomas, the distance between the outer peripheral edge of the myoma to the serosa should be measured for consideration of surgical resection.
Qualitative Assessment of the Endometrium
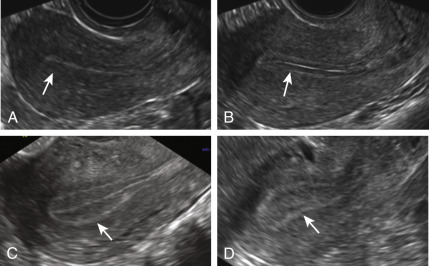
The endometrium consists of three layers: stratum basalis (basal layer), stratum compactum, and the stratum spongialis. The stratum compactum and stratum spongialis develop into the stratum functionalis (functional layer) during the proliferative phase of the menstrual cycle. The appearance of the endometrium on ultrasound varies in a relatively characteristic pattern throughout the cycle. The endometrium is relatively thin, 3 to 5 mm, in the postmenstrual early follicular phase of the cycle and is a single, hyperechogenic line ( Fig. 35.7 ). The endometrium increases in thickness throughout the follicular phase of the menstrual cycle in response to endometrial proliferation induced by E 2 and is accompanied by the induction of ER and PRs. The endometrium gradually thickens with the functional and basal layers visually differentiated during the mid-to-late follicular phase (see Fig. 35.7 ). In the late follicular phase and periovulatory period, the endometrium assumes a trilaminar appearance with a central echogenic line of opposing hypoechogenic functional layers and slightly hyperechoic basal layers more peripherally. Mean preovulatory trilaminar EMT measurements vary based on stimulation protocol used for the ovulation induction; however, the measurements are approximately 8 mm in spontaneous cycles, 9 to 10 mm in cycles with oral induction agents, and approximately 12 mm in cycles stimulated with exogenous gonadotropins. In the luteal phase of the menstrual cycle, the endometrium appears homogeneous, hyperechoic with the endometrial glands branching and expanding under the influence of progesterone. In pregnancy, the echogenicity and EMT are maintained as decidual reaction progressed to implantation. In the absence of pregnancy, with a sharp decline in progesterone levels due to demise of the corpus luteum, the endometrium regresses with shedding of the functional layer.
EMT is a biomarker for endometrial receptivity, yet EMT in itself does not reflect endometrial function. Investigation into the molecular, proteomic, and genetic aspects of endometrial physiology have provided interesting information with regard to the window of implantation, but are not yet clinically applicable. Many reports have analyzed the correlation between EMT and the ability of embryos to implant, and there is general consensus that the higher the EMT (>9 mm), the better the chance of implantation. EMT and sonographic echogenic patterns have been compared with pregnancy rates in in vitro fertilization (IVF) cycles, with most authors agreeing that a thin EMT, defined as 7 mm or less, is correlated with a lower implantation rate. A systemic review and meta-analysis of 22 studies was published in 2014 and reported a virtually absent discriminatory capacity of EMT in the prediction of occurrence of pregnancy in ART cycles (pregnant vs. not pregnant). A trend towards lower ongoing pregnancy and live birth rates for women with EMT 7 mm or less was observed (OR, 0.38; 95% CI, 0.09 to 1.5), with the probability of a clinical pregnancy for an EMT 7 mm or less significantly lower compared with cases with EMT greater than 7 mm (23.3% vs. 48.1%; OR, 0.42; 95% CI, 0.27 to 0.670). Another observation of the authors in the meta-analysis was that the overall incidence of an endometrial echo thickness of less than 7 mm is low (2.4%) compared with the overall rate of implantation failure, and thus a thin endometrium may explain implantation failure in only a small subset of patients. These authors suggested clinicians refrain from clinical decisions based solely on EMT measurement.
While a thin endometrium increases the likelihood of no conception, it does not eliminate the chance for pregnancy. Others have described implantation with minimum EMT threshold values of 4 to 5 mm, demonstrating a thin endometrium does not necessarily exclude the chance of pregnancy. The etiology for low conception rates with a thin endometrium is poorly understood, but endometrial vascular function is considered to be important in blastocyst implantation. It has been proposed that a thin endometrium would expose an implanting embryo closer to the spiral arteries and the alteration in oxygen tension would be detrimental to the implanting blastocyst. Severe treatments have been proposed to encourage endometrial proliferation at the time of ART cycles in those patients with low EMT, including extended estrogen, aspirin, granulocyte-colony stimulating factor, vaginal sildenafil citrate (Viagra), pentoxifylline, and tocopherol (vitamin E). As a rule, data are contradictory or based on too few individuals to support any specific treatment regimen.
Endometrial receptivity may be reflected by the ultrasonographic endometrial echo pattern, but meta-analysis is hampered because different classification systems to describe these echo patterns have been utilized in independent studies. Some studies found an association between endometrial pattern and IVF outcome, while other studies show no significant correlation. A tri-laminar endometrial echo pattern on the day of hCG administration is associated with higher clinical pregnancy rates in IVF cohorts, but not necessarily throughout the range of endometrial echo thickness.
Three-dimensional (3D) TVS is increasingly common in ART. This technique provides accurate contrast between the myometrium and the endometrium for calculating an endometrial volume, or a specific targeted portion of the total volume within the endometrial cavity. Volume calculation by 3D US is performed utilizing a post image acquisition computer aided program—Virtual Organ Computer-Aided Analysis (VOCAL)—a combination of voxels and geometric information of surfaces in a 3D data set. VOCAL has been utilized with high reproducibility and intraobserver reliability for OV, power Doppler indices, and endometrial volume. Total endometrial volume as calculated may be a more accurate assessment of endometrial receptivity, but to date, studies have documented a range of volume thresholds (<1 mL to 3.9 mL) under which conception does not occur. Yet again, too few individuals to date have been included to make a definitive assessment of the use of 3D endometrial volume as a predictor of pregnancy in ART.
The definitions suggested to qualitatively assess the endometrium in daily practice by the IETA are noted in Table 35.2 . In suggesting standardized terminology, the study group hoped to facilitate data comparisons between future studies and facilitate multicenter studies. While EMT may be an important marker for pathology, endometrial morphology may prove equally as useful. An evaluation of endometrial morphology includes an assessment of the endometrial echogenicity, characteristics of the endometrial midline, and descriptors of the endometrial-myometrial junction. The echogenicity of the endometrium is described as hyper-, hypo-, or isoechoic, as compared with the echogenicity of the myometrium, and “uniform” (homogeneous with symmetrical anterior and posterior sides) and includes the tri-layer pattern, as well as homogeneous hyperechogenic, hypoechogenic, and isoechogenic endometrium. The echogenicity is defined as nonuniform if the endometrium appears heterogeneous, asymmetrical, or cystic. The endometrial midline is “linear” (straight with a clear interface is detected), “nonlinear” if a waved interface is visualized, and “irregular” or “not defined” in the absence of a distinct interface. A “bright edge” is the echo formed by the interface of an intracavitary lesion and the surrounding endometrium. The endometrial-myometrial junction is described as regular, irregular, interrupted, or not defined. Synechiae are defined as strands of tissue crossing the endometrium, while intracavitary fluid is described as anechogenic or low-level echogenicity, “ground glass,” or mixed echogenicity.
Color and Power Doppler Assessment
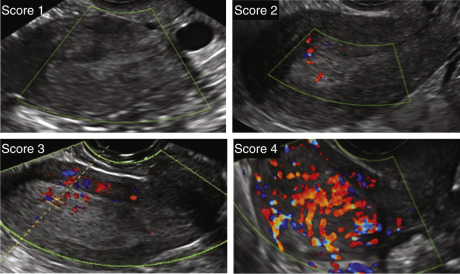
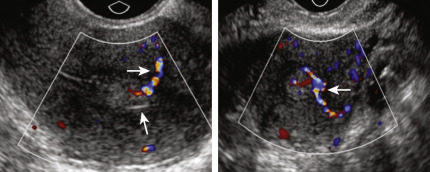
The IETA standard definitions describe color and power Doppler assessment of the endometrium and immediate surrounding myometrium. An image of the midsagittal section of the uterus should be magnified with the color and power Doppler box placed to include the endometrium and the surrounding myometrium. The suggested settings (ultrasound frequency of at least 5.0 Mhz, pulse repetition frequency 0.3 to 0.9 kHz, wall filter 30 to 50 Hz, color power Doppler gain reduced until all color artifacts disappear) ensure maximum sensitivity to blood flow. The color score is a subjective semiquantitative assessment of the amount of blood flow present and is based on color content score first suggested by the International Ovarian Tumor Analysis (IOTA) color score applied previously to ovarian masses. The color score is 1 (not zero by convention) when there is no flow within the endometrium, a score of 2 when minimal color can be detected, a score of 3 when moderate color is present, and a score of 4 when abundant color is detected ( Fig. 35.8 ).
In the IETA endometrial terminology, the vascular pattern is then further defined within the endometrium with respect to the presence or absence of “dominant vessels,” or of another specific vascular pattern. “Dominant vessels” are defined as one or more distinct vessels (arterial and/or venous) traversing the endomyometrial junction. The dominant vessel may show branching within the endometrium and is further characterized as either orderly or disorderly/chaotic. Dominant vessels may present as a single vessel (formerly known as the “pedicle artery sign”) with or without branching. Multiple dominant vessels may be “focal origin,” or “multifocal origin,” at the endometrial-myometrial junction. Lastly, other vascular patterns within the endometrium include scattered vessels (dispersed color signals within the endometrium but without visible origin at the myometrial-endometrial junction), and circular flow.
Sonohysterography or Saline Infusion Sonography: Enhanced Ultrasound of the Uterine Cavity
Qualitative Assessment of Endometrial Morphology and Intrauterine Lesions at Sonohysterography
SHG, or saline infusion sonohysterography (SIS), is the instillation of isotonic saline into the uterine cavity to create greater contrast to delineate and outline intracavitary filling defects and uterine shape abnormalities ( ). It is a simple, accurate, well-tolerated, efficient outpatient tool in the assessment of endometrial cavity pathologies such as endometrial polyps, submucous myomas, intrauterine adhesions, and congenital uterine anomalies. In a recent systematic review conducted of 20 studies comparing SHG with hysteroscopy in the detection of all intracavitary abnormalities in the subfertile woman, Seshadri et al. reported a pooled sensitivity of SHG of 0.88 (95% CI, 0.85 to 0.90), and a pooled specificity of 0.94 (95% CI, 0.93 to 0.96). The positive and negative likelihood ratios were 20.93 (95% CI, 9.06 to 48.34) and 0.15 (95% CI, 0.10 to 0.22), respectively. SHG had good accuracy in the detection of all intrauterine abnormalities (area under the summary receiver operating curve [sROC] = 0.97 ± 0.01). SHG also had a high-pooled sensitivity and specificity in the detection of congenital uterine anomalies, 0.85 (95% CI, 0.79 to 0.90) and 1.00 (95% CI, 0.99 to 1.00), respectively. These results are consistent with those of de Kroon et al., who performed a systematic review and meta-analysis of 24 studies comparing SHG and surgical findings (hysteroscopy or hysterectomy) in pre- and postmenopausal women suffering with abnormal uterine bleeding (AUB). In this analysis, the pooled sensitivity and pooled specificity of SHG in uterine cavity evaluation for AUB were 0.95 (95% CI, 0.93 to 0.97) and 0.88 (95% CI, 0.85 to 0.92), respectively; the likelihood ratios were, respectively, 0.91 (95% CI, 0.89 to 0.94) and 0.07 (95% CI, 0.04 to 0.10). The overall success rate of performance of an SHG was 93% (95% CI, 0.92 to 0.94), with the success rate among postmenopausal women significantly lower ( P < .01; success rate 86.5%; 95% CI, 0.832 to 0.899) than that of the success rate of premenopausal women (success rate 95%, 95% CI 0.94 to 0.96). In this study, the 7% of uterine cavity abnormalities undetected by SHG as compared with hysteroscopy or hysterectomy were polyps, perhaps small, the clinical significance of which is unknown.
Indications for SHG include but are not limited to the evaluation of AUB; assessment of intracavitary polyps, fibroids, and synechiae; assessment of focal and diffuse endometrial abnormalities; and congenital abnormalities of the uterus, in the assessment of the subfertile patient and in those with recurrent pregnancy loss. SHG should not be performed in a woman who is pregnant. In cases of intermittent or prolonged AUB, treatment with a short course of progestin may enable appropriate timing of the procedure. SHG should not be performed in patients with existing pelvic infection, unexplained pelvic tenderness, or if pelvic infection is suspected. The routine use of antibiotic prophylaxis is not recommended, but consideration should be given to administering antibiotics based on individual risk (e.g., the presence of nontender hydrosalpinges or a past history of pelvic inflammatory disease). A pregnancy test is advised when clinically indicated. Active vaginal bleeding is not a contraindication to the procedure but may make the interpretation more challenging due to clots often mimicking focal lesions.
Technique of Sonohysterography
A complete transvaginal ultrasound should be performed prior to SHG. The cervix is visualized and prepped with an antiseptic solution such as povidone-iodine or chlorhexidine gluconate solution. A variety of suitable catheters are available, with small diameter catheters; some with a stylet, which may be helpful for the small os and in cases of cervical stenosis; and some with a balloon or acorn catheter, in the presence of a patulous cervix, a uterus enlarged with fibroids, and when tubal patency assessment is a goal of the study. Saline has been the traditional distension media of choice for uterine cavity evaluation. A 10-mL syringe prefilled with saline is used to preflush the catheter to remove all air from entering the cavity and contributing to image distortion. If using a balloon catheter, fill the balloon with saline to avoid acoustic distortion.
More recently the use of gel infusion sonohysterography (GIS) has gained interest and has been demonstrated to be equal to saline infusion with respect to clinical feasibility in the assessment of the uterine cavity. Gel infusion sonography is performed with either a 2.0-mm neonatal suction catheter or insemination catheter through which a sterile gel preparation of hydroxyethyl cellulose and glycerol with chlorhexidine and lidocaine (2 g in 100 g of gel) (Instillagel, FARCO-PHARMA GmbH, Köln, Germany), or without lidocaine (Endosgel, FARCO-PHARMA GmbH). These gels have long been used for vaginal, intrauterine, and intraurethral instillation prior to cystoscopy and have been reported as safe. Hydroxyethyl cellulose and glycerol are considered to be safe in cases of intravasation or intraabdominal application. Some authors have suggested utilizing hydroxyethyl cellulose gel without chlorhexidine and lidocaine, citing no additional value of either of these substances. Gel has additional useful physical properties; it offers the same negative contrast as saline, but its viscosity is much higher than saline, resulting in less backflow through the cervix and more stable filling of the uterine cavity, and thus obviating the need for a more expensive balloon catheter. Because of its higher viscosity and smaller instillation volume (range 2 to 10 mL as compared to 5 to 20 mL), trans-tubal spillage is less likely to occur. The gel’s high viscosity can be modulated for instillation through a 2-mm thin catheter by warming it to 37°C. Disadvantages of GIS are blood clots and debris, which are not flushed out by the gel and may mimic a polyp or myoma. In a prospective observational cohort study including 551 consecutive patients with AUB, Van den Bosch et al. found GIS (149 women) and SHG (402 women) similar techniques in terms of technical feasibility, and with no significant difference in the detection of focal endometrial lesions as compared with those findings determined at surgical pathologic evaluation.
Evaluation of the endometrial and intracavitary morphological features at SHG is described as noted in Table 35.3 . Uterine distention at SHG is defined as “optimal” if fluid or gel clearly distends the cavity, “suboptimal” if the cavity is barely distended, and “failed” if the cavity cannot be distended with fluid. The EMT is measured on either side of the fluid, with the fluid excluded, and the total thickness reported as the total of the anterior and posterior endometrial measurement, yet each individual thickness is noted and recorded. The endometrial outline is defined as smooth if the endometrial surface facing the endometrial cavity appears regular, as having endometrial folds (multiple thickened “undulating” areas, “moguls” with a regular profile), or as “polypoid” if there are deep indentations ( Fig. 35.9 ). The endometrium is described as “irregular” if the surface facing the uterine cavity is cauliflower-like or sharply toothed (“spiky”). The endometrial-myometrial junction is described as noted in Table 35.3 .
| Sonohysterography (or Spontaneous Intracavitary Fluid) | Descriptor |
|---|---|
| Endometrial thickness | Optimal/suboptimal/failed |
| Endometrial outline | Smooth, endometrial folds, polypoid, irregular |
| Intracavitary lesion | No/Yes and number |
| Endometrial lesion(s) | Extent: localized (<25%), extended (≥25%), or not assessable |
| Type of localized lesion(s) | Pedunculated/sessile/not applicable/not assessable |
| Echogenicity | Uniform: hyperechoic/hypoechoic/isoechoic, or Nonuniform: without cystic areas or with regular or irregular cystic areas |
| Outline | Regular/irregular |
| Myometrial derived lesion(s) | Echogenicity: uniform/nonuniform |
| Grading: G0 (within the cavity), G1 (endocavitary part ≥50%), G2 (endocavitary part <50%) | |
| Measurement of lesion: d1 x d2 x d3 in mm | |
| Color Doppler assessment | Color score within the endometrium: 1 (no flow); 2 (minimal flow); 3 (moderate flow); 4 (abundant flow) |
| Vascular pattern: no vessels/single “dominant” vessel with or without branching/multiple “dominant” vessels focal origin or multifocal in origin/scattered vessels/circular flow |

Any intracavitary lesion with anything that protrudes into the uterine cavity on SHG or GIS is by convention called an intracavitary lesion, and is further described as either endometrial or lesions arising from the myometrium. The extent of the endometrial lesion is estimated subjectively by the sonographer and is classified as “extended” if it involves 25% or more of the endometrial surface, or “localized” if less than 25%. A “localized” endometrial lesion is either “pedunculated” (diameter of the base/maximum transverse diameter of the lesion) if less than 1, or “sessile” if the diameter of the base/maximum diameter of the lesion is 1 or more. The outline of the lesion within the cavity is described as “regular” or “irregular” (e.g., spiky or cauliflower-like).
The presence of intracavitary lesions arising from the myometrium (most commonly myomas) is defined by the grade (proportion of the myoma extending into the cavity) and the echogenicity. Submucosal (SM) myomas are graded according to the description of Leone et al., G0: pedunculated myoma, completely intracavitary, and lacking in intramural extension; G1: submucous myoma with intramural extension less than 50%; and G2: SM myoma with intramural extension higher than 50% ( Fig. 35.10 ). The echogenicity of the lesion is described as “uniform” or “nonuniform.” Synechiae are thin or thick strands of tissue crossing the endometrial cavity and may be the same echogenicity as the myometrium, and are attached to both uterine walls. Often the uterine cavity will not distend completely at SHG in the presence of synechiae ( Fig. 35.11 ). The color score (subjective assessment from 1 to 4) is outlined under the unenhanced ultrasound examination.
PALM-COEIN
- ◆
PALM-COEIN is a system for the classification of the potential causes of AUB as a means of defining a consistent and universally accepted nomenclature.
- ◆
Three-dimensional ultrasound coronal views of the uterus with and without saline enhancement will give more detail to uterine cavity structural abnormalities that may contribute to bleeding, and a more detailed assessment of the extent of adenomyosis.
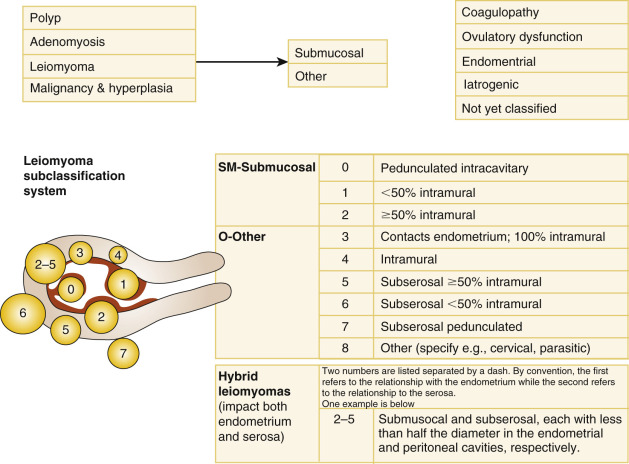
In 2011, the working group for the International Federation of Gynecology and Obstetrics (FIGO) proposed a new system for the classification of the potential causes of AUB as a means of defining a consistent and universally accepted nomenclature to be used by clinicians and investigators ( Fig. 35.12 ). The PALM-COEIN ( p olyp; a denomyosis; l eiomyoma; m alignancy and hyperplasia; c oagulopathy; o vulatory dysfunction; e ndometrial; i atrogenic; and n ot yet classified) classification system of AUB in nongravid women of reproductive age was approved by the FIGO executive board and adopted by the American College of Obstetricians and Gynecologists. The PALM-COEIN system classifies uterine bleeding abnormalities by bleeding pattern and etiology.
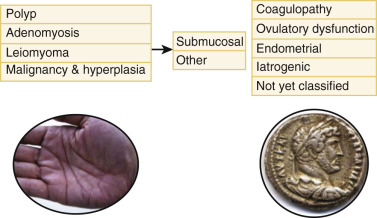
The terms menorrhagia and dysfunctional uterine bleeding were discarded in favor of the overarching term AUB , which may include menstrual bleeding that is abnormally heavy, and may also include bleeding that is abnormal in timing. The term heavy menstrual bleeding (HMB) replaced the term menorrhagia for the symptom of excess menstrual bleeding. AUB is further classified by one (or more) letter qualifiers that indicate the etiology. Of the nine main categories within the PALM-COEIN (pronounced “pahm-koin”), four are discrete, structural entities that can be measured visually with imaging techniques and/or histopathology. The remaining entities within the COEIN group are nonstructural. The classification system was constructed, recognizing that any patient could have one or several entities contributing to AUB, or have defined structural abnormalities and be asymptomatic.
Polyp (AUB-P)
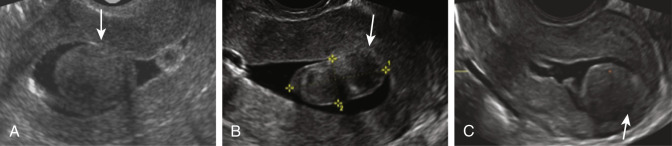
Endometrial polyps are epithelial proliferations that comprise a variable proportion of vascular, glandular, fibromuscular, and connective tissue components arising from the surface of the endometrium. They may be sessile or pedunculated. The ultrasound features of an endometrial polyp include a hyperechogenic area within the endometrial cavity, with or without regular small cysts, surrounded by a “bright edge” ( Fig. 35.13 ). The bright edge is explained by the interface between the polyp and the surrounding endometrium. Endometrial polyps are known to contain thick-walled dilated blood vessels within the glandular tissue and fibrous stroma. When a polyp is suspected, color Doppler imaging of the endometrium may detect a central vessel visualized to enter the endometrium from the surrounding myometrium, assigned the descriptive term pedicle artery sign, and has also been called a feeding vessel. In women with postmenopausal bleeding (PMB), Timmerman et al. found the pedicle artery sign to have a sensitivity of 76.5%, a specificity of 95.3%, a positive predictive value of 81.3%, and a negative predictive value of 93.8%. Alternatively, Alcazar et al. reported the presence of a single vessel penetrating the endometrium from the myometrium to have a sensitivity of 97% (33/34), endometrial polyps, and a specificity of 86% (42/49). Endometrial polyps may be asymptomatic and are typically benign growths. Polyps may contain foci of malignancy. In a retrospective multicenter study, Ferrazzi et al. reported a single case of stage 1 grade 1 endometrial cancer (EC; 0.1%) in a 40 mm polyp out of 1152 asymptomatic women who underwent hysteroscopic polypectomy, a prevalence 10 times lower than that observed in patients presenting with PMB. The prevalence of atypical hyperplastic polyps was significantly lower, 1.2% in asymptomatic women, than those patients presenting with PMB (2.2%; P < .05). In further evaluation of their data, these authors suggest the only variable associated with abnormal histology (cancer, polypoid cancer, and atypical hyperplasia) in asymptomatic women was a mean polyp diameter of greater than 18 mm.
Adenomyosis (AUB-A)
Adenomyosis is a common gynecologic disorder characterized by the migration of endometrial glands and stroma from the basal layer of the endometrium into the myometrium and is associated with surrounding hypertrophic and hyperplastic myometrium. Estimates of the prevalence of adenomyosis vary widely, ranging from 5% to 70%, with the variation in prevalence related to the criteria used for diagnosis at histopathologic assessment, the technique used to obtain the myometrial samples, and the characteristics of the patient population studies.
Adenomyosis may be asymptomatic in about 30% of cases; the remaining women report the most frequent symptoms of menorrhagia (50%), dysmenorrhea (30%), and HMB (20%). The relationship between adenomyosis and the genesis of AUB is unclear. The definitive diagnosis of adenomyosis is based on histologic exam after hysterectomy. By tradition, a histologic diagnosis is made when endometrial glands and stroma are found at least one low-power field beneath the endomyometrial junction (≥4 mm). Beyond these criteria, there are no universally accepted criteria to establish the diagnosis of adenomyosis with definitions of “foci located deeper than 25% of the myometrial thickness” or “glandular extensions greater than 1 to 3 mm below the endometrial layer” commonly used. Most studies use a cutoff of 2.5 mm below the basalis layer to define the minimal depth of invasion.
Transvaginal ultrasound and MRI both demonstrate high levels of accuracy in the preoperative diagnosis of adenomyosis. The accuracy of 2D TVS is comparable with that of MRI and/or histology, with a sensitivity of 75% to 88% and a specificity of 67% to 93%. The 2D TVS features of adenomyosis are alterations in the deep myometrium, whereas MRI and 3D TVS have specific signs within the JZ, especially with post volume acquisition image processing. Features consistent with adenomyosis on 2D ultrasonography include an enlarged, globular uterus with round cystic areas, either hypoechoic or hyperechoic, within the myometrium ( Figs. 35.14 and 35.15 ). In focal adenomyosis, the fundus may be asymmetrically enlarged (the anterior wall may be thicker than the posterior wall), with an inhomogeneous tissue, with irregular myometrial echotexture in an indistinctly defined myometrial area with either an increased or decreased echogenicity. Linear striations with thin acoustic shadows may be visualized, radiating from the echogenic focus within the myometrium where no distinct leiomyoma is seen. The endometrial-myometrial border is described as indistinct or “fuzzy,” and the endometrial echo complex may be ill-defined. The vascular pattern within the myometrium is diffuse with small vessels that are haphazard and do not follow the normal course of the arcuate or radial arteries. The presence of two or more of these findings on 2D TVS is considered diagnostic ( Table 35.4 ).
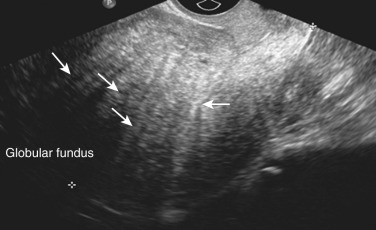
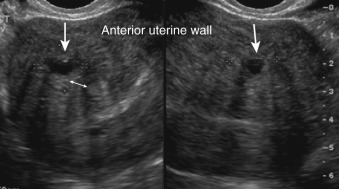
| Globally enlarged uterus: the fundus of the uterus appears to be enlarged |
| Asymmetrically enlarged uterus: anterior wall thicker than the posterior wall, unrelated to myomas |
| Myometrial cysts: rounded cystic area within the myometrium with power Doppler used to distinguish myometrial cysts from blood vessels |
| Inhomogeneous and irregular myometrial echotexture in an indistinctly defined myometrial area with decreased or increased echogenicity; hyperechogenic islands, subendometrial lines, and buds |
| “Fan-shaped shadowing”: Myometrial hypoechoic linear striations seen as a radiating pattern of thin acoustic shadows not arising from echogenic foci or leiomyomas |
| Indistinct, fuzzy endometrial-myometrial border (ill-defined endometrial stripe) |
| Presence of diffuse minimal vascularity seen as diffuse spread of small vessels without the normal course of the arcuate or radial arteries inside the myometrium |
| “Question Mark Sign”: corpus flexed backward, the fundus of the uterus faces the posterior pelvic compartment, and the cervix is directed frontally toward the uterine bladder |
Three-Dimensional Transvaginal Sonographic Features of Adenomyosis
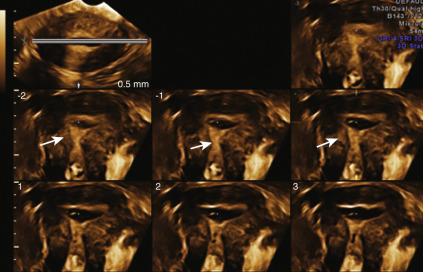
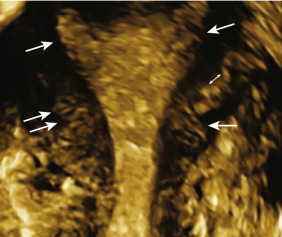
Three-dimensional TVS assessment of adenomyosis is based on the evaluation of the JZ on the acquired volume of the uterus obtained in a standardized multiplanar view (sagittal, transverse, and coronal planes), thus creating an optimized rendered coronal uterine cavity view. On 3D TVS, the JZ can be evaluated more clearly and appears as a dark halo of tissue surrounding the endometrium. In the presence of adenomyosis, the JZ is indistinct with distortion and infiltration of hyperechoic endometrial tissue into the JZ. According to the IETA and MUSA criteria (see Tables 35.2 and 35.3 ), the JZ may be regular, irregular, interrupted, not visible, not assessable, or many manifest with more than one feature. Any irregularity in the JZ (e.g., hyperechoic buds and lines, cystic areas) and their location within the uterus should be described ( Fig. 35.16 ).
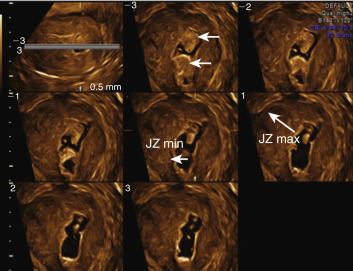
In addition to the subjective morphologic evaluation of the JZ, objective parameters such as thickness of the JZ (representative of the severity of adenomyosis), similar to those reported in MRI imaging, have been proposed. A maximal thickness of the JZ (JZmax) is measured in the area where the JZ appears to be the thickest, and the minimal thickness (JZmin) is measured at the thinnest portion. A total myometrial thickness is measured perpendicular to the endometrium on the same section. The magnitude of the JZ irregularity, and thus severity of the adenomyosis, is expressed as the difference between the maximal and minimal JZ thickness: JZmax − Jzmin = JZdiff ( Fig. 35.17 ). The extent of the JZ irregularity can be reported as the subjective estimation of the percentage of the JZ that is irregular (<50% or ≥50%). It has been observed that pelvic endometriosis, especially in advanced stages, are strongly associated with JZ thickening and adenomyosis.
Leiomyoma (AUB-L)
On ultrasound, uterine fibroids appear as solid, well-defined round lesions within the uterine corpus, and rarely within the cervix. The myomas often have an inhomogeneous echo-architecture, may be hypo- or hyperechoic, and characterized with variable degrees of acoustic shadowing and refractive artifacts ( Fig. 35.18A and B ). The echogenicity of the lesion varies with cellular content of muscle, fibrous stroma, calcification, and the degree of cystic, lipomatous, hemorrhagic, or hyaline degeneration. Myomas associated with fatty degeneration (see Fig. 35.18C ), and those with dense calcium deposits, appear hyperechoic. Myomas typically demonstrate diffuse, but mostly peripheral flow on color or power Doppler ( Fig. 35.19 ). Both TAS and TVS may be needed to adequately evaluate the uterus; large or pedunculated myomas may be missed by TVS alone. A pedunculated myoma may be suspected when a solid pelvic mass is visualized, separate from the ovaries, and demonstrates a narrow vascular stalk with Doppler flow extending from the uterus to an isoechoic mass (see Fig. 35.18D ). These pedunculated masses may “slide” against the ultrasonographic probe.
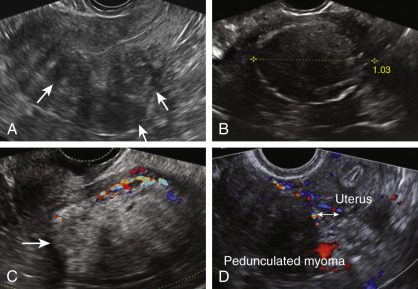
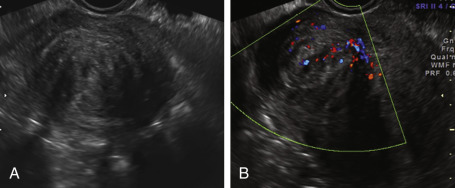
Given the prevalence of these lesions, up to 70% in Caucasians and up to 80% in women of African ancestry, they may be present and asymptomatic, and thus not causal of the patient’s AUB. Myomas are classified as intramural, subserosal, subendometrial, or a combination of these. In the PALM-COEIN classification system, the clinician is required to (1) document the presence of absence of 1 or more myomas; (2) distinguish leiomyomas involving the endometrial cavity (SM) from others (O), because it is generally held that SM lesions are more likely to contribute to the etiology of AUB; and (3) categorize intramural, subserosal, and possible parasitic lesions based on the criteria of Wamsteker and accepted by the European Society of Human Reproduction and Embryology (ESHRE; Fig. 35.20 ).
The mechanism of how leiomyomata contribute to HMB is not completely understood. There is a fourfold increase in the expression of plasminogen activator inhibitors (PAI), a reduction of antithrombin III and thrombomodulin within the endometrium of women that have fibroid associated HMB. Leiomyoma secrete transforming growth factor (TGF-β), a cytokine that acts as a stimulator of angiogenesis. The location of myomata plays a role in the likelihood of development of HMB. SM and intrauterine myomas, which increase the endometrial surface area available for withdrawal bleeding, are more commonly associated with HMB.
Malignancy and Hyperplasia (AUB-M)
Although EC is thought to be a cancer of the postmenopausal years, 14% of cases are diagnosed in premenopausal women, and 5% of who are younger than 40 years. Premalignant hyperplastic and malignant processes of the endometrium arising during the investigation of women of reproductive age with AUB is classified as AUB-M. The PALM-COEIN classification system for AUB-M in reproductive age women did not seek to replace the 1994 World Health Organization (WHO) 1994 and FIGO classification systems for “endometrial hyperplasia and neoplasia.”
The 94WHO classification system has been widely used and segregates endometrial hyperplasia by architecture (simple vs. complex) and cytology (atypical vs. nonatypical). This four-part category system (simple hyperplasia, complex hyperplasia, simple hyperplasia with atypia, and complex hyperplasia) is limited by poor diagnostic reproducibility, the four separate categories do not correspond to distinct biologic entities, and the individual categories do not suggest specific management algorithms. “Endometrial hyperplasia” is a term that has historically been implemented with several qualifiers to encompass both clonal premalignant lesions and benign field effects of the endometrium in response to prolonged and/or excessive estrogen exposure.
In the schema developed by the International Endometrial Collaborative Group, histomorphologic, genetic, clinical, and biological data were used to develop quantitative pathologic criteria for three disease categories: (1) benign endometrial hyperplasia, (2) endometrial intraepithelial neoplasia (EIN; premalignant), and (3) endometrial adenocarcinoma, endometrioid type, well differentiated (malignant; Table e35.4 ). A diagnosis of EIN is rendered when a lesion has a minimum dimension of 1 mm, the area of glands exceeds the area of stroma, the cytology is changed relative to background, and both benign mimics (polyps, secretory endometrium, and effects of exogenous estrogen) and cancer can be excluded ( Table e35.5 ). It has been demonstrated that clinical outcome prediction and interobserver reproducibility with the EIN classification system is greater than the 94WHO schema.
| Nomenclature | Topography | Functional Category | Treatment |
|---|---|---|---|
| Benign endometrial hyperplasia | Diffuse | Prolonged estrogen effect | Hormonal therapy, symptomatic |
| Endometrial intraepithelial neoplasia | Focal progressing to diffuse | Precancerous | Hormonal therapy or surgery |
| Endometrial adenocarcinoma, endometrioid type, well-differentiated | Focal progressing to diffuse | Malignant | Surgery, stage-based |
| Endometrial Intraepithelial Neoplasia Criteria | Comments |
|---|---|
| Architecture | Area of glands greater than stroma (volume percentage stroma less than 55%) |
| Cytology | Cytology differs between architecturally crowded focus and background |
| Size greater than 1 mm | Maximum linear dimension exceeds 1 mm |
| Exclude mimics | Benign conditions with overlapping criteria (i.e., basalis, secretory, polyps, repair |
| Exclude cancer | Carcinoma if maze-like glands, solid areas, or appreciable cribriforming |
True benign endometrial “hyperplasias” are polyclonal proliferations involving endometrial glands and stroma that develop in response to the systemic effects of an abnormal estrogenic stimulus. The morphology of these benign endometrial proliferations changes with duration and dose of exposure and is dependent upon the patient and her medical history. These polyclonal proliferations are categorized in the EIN schema as “benign endometrial hyperplasia.”
As the majority of patients with hyperplasia and EC will present with PMB, the imaging characteristics of EIN and endometrial carcinoma will be addressed in the section of PMB.
Coagulopathy (AUB-C)
Deficiencies in coagulation factors or abnormalities in platelet function are commonly associated with abnormally excessive or prolonged uterine bleeding. Undiagnosed bleeding disorders, in particular von Willebrand disease (vWD) and platelet function disorders, can be an underlying cause of HMB. In a recent systemic review incorporating 11 studies, an overall prevalence of 13% of vWD was reported in t > 900 women presenting with HMB. There was even a higher prevalence, up to 20% in adolescents presenting with acute HMB at menarche. vWD is characterized by deficient or defective von Willebrand factor (vWF), a glycoprotein that promotes platelet adhesion to vessel walls after vessel injury. vWF plays a critical role in platelet adhesion and platelet plug formation during primary hemostasis and serves as a carrier protein for factor VIII. Deficiencies in vWF may result in mucosal bleeding in the oropharynx, gastrointestinal, and genitourinary tract in all patients, and in women, HMB is a common symptom in up to 80%.
HMB is also reported in high prevalence in carriers of hemophilia and disorders of platelet function. Chronic liver disease results in a global deficiency of the clotting factors synthesized by the liver. Women with HMB on chronic therapeutic anticoagulation also fall within this category. HMB is a relatively common occurrence with the use of chronic anticoagulation (low-molecular-weight heparin, heparin, and warfarin). The attributable mechanism of HMB in this instance is the impairment of the formation of an adequate “plug” or “clot” within the vascular lumen. Women using such agents essentially have a systemic disorder of hemostasis. Although in this case the AUB could be considered iatrogenic and classified accordingly, Munro and colleagues determined it would be more appropriate to classify women affected with HMB associated with chronic anticoagulation use as having a coagulopathy (AUB/HMB-C). There are few available studies of platelet disorders, including thrombocytopenia in the acute setting of menorrhagia, and thus the prevalence may be underestimated. Based on the reports available, immune thrombocytopenia purpura (ITP) may be one of the most common causes of acute menorrhagia in adolescents.
Ovulatory Dysfunction (AUB-O)
Anovulatory bleeding is common in the extremes of the reproductive life span, a common etiology in adolescence and in the perimenopausal transition. The bleeding pattern associated with ovulatory dysfunction can manifest as unpredictable timing and varying in the amount of flow to HMB. The disorders of ovulation may present as a spectrum of menstrual abnormalities, ranging from amenorrhea and extremely light and infrequent bleeding to episodes of unpredictable and extremely HMB requiring medical or surgical intervention. During the first 12 to 18 months after the onset of menstruation, the immaturity of the hypothalamic-pituitary axis frequently is the cause of AUB-O. Obesity is becoming an important comorbid condition in the evaluation of the adolescent with AUB-O. Although many disorders of ovulatory function elude a defined etiology, the differential diagnosis includes excessive exercise, obesity, hypothalamic suppression related to stress, anorexia, hypothyroidism, hyperprolactinemia, and premature ovarian insufficiency. In some instances of AUB-O, the ovulatory dysfunction may be related to the use of gonadal steroids or medications that impact the central dopaminergic metabolism by reducing serotonin uptake such as phenothiazines and tricyclic antidepressants. It has been proposed that the resulting reduced inhibition of PRL release causes prolactin-related disruption in the hypothalamic-pituitary-ovarian axis and impacts ovulatory function.
Endometrial (AUB-E)
The normal cessation of menstrual bleeding is achieved by endometrial hemostasis via platelet aggregation, fibrin deposition, and thrombus formation. Local endocrine, immunological, and hemostatic factors interact at the molecular level to control endometrial hemostasis. Abnormalities of uterine bleeding can result from an imbalance of hemostatic factors. Tissue factor (TF) and thrombin play a key role locally in the cessation of menstrual bleeding through the activation of factors involved in coagulation. Alternatively, fibrinolysis prevents clot organization within the uterine cavity, while PAI and thrombin-activatable fibrinolysis inhibitors control plasminogen activators and plasmin activity.
HMB, without another identifiable cause, may be a primary disorder of endometrial hemostasis. The development of antifibrinolytics in the treatment of AUB is based on the understanding of the local hemostatic factors within the endometrium. Tranexamic acid (TA; amino-methyl-cyclohexane-carbolic acid) is such an agent. In women with HMB, treatment with TA was associated with a 54% reduction in menstrual blood loss and is known to be associated with a significant reduction in endometrial tissue plasminogen activator levels.
Iatrogenic (AUB-I)
Unscheduled uterine bleeding that occurs as a result of the use of medication, or an inert intrauterine system, is termed AUB-I. This category includes prescribed therapy that directly impacts the endometrium, interferes with coagulation mechanisms, or influences the process of ovulation. Gonadal steroids-estrogens, progestins, and androgens act systemically by influencing the action of the central access, the ovary, and have a direct impact on endometrial homeostasis. Other potential causes of iatrogenically induced bleeding include anticonvulsants and antibiotics that may alter the circulating levels of endogenous steroid hormones. Unscheduled bleeding in the first 3 to 6 months of use of the levonorgestrel IUS is a common complaint. Other medications that impact the central axis have been discussed under the section AUB-O.
Not Yet Classified (AUB-N)
The intent of the AUB-N within the PALM-COEIN system is to provide a category for entities that have not been shown conclusively to contribute to AUB—for example, chronic endometritis, arteriovenous malformations (AVMs), and myometrial hypertrophy—and for those entities not yet identified that might contribute to AUB.
Acute Versus Chronic Abnormal Uterine Bleeding
AUB can be further classified as acute or chronic. Acute uterine bleeding is defined as AUB in nonpregnant women that, in the opinion of the clinician, is of sufficient quantity to require immediate intervention to prevent further blood loss. Acute AUB can be defined as HMB or intermenstrual bleeding (IMB), occurring in a woman of childbearing age requiring emergency treatment excluding pregnancy, postpartum hemorrhage, trauma, or malignancy. Acute AUB can occur as a new event or may occur in the setting of chronic AUB. Chronic AUB is defined as bleeding from the uterine corpus that is abnormal in volume, regularity, and/or timing, and has been present for the majority of the past 6 months. IMB is defined as bleeding between clearly defined cyclic and predictable menses.
Pelvic ultrasound is an essential tool in the investigation of the patient with AUB, with the laboratory and imaging investigation tailored to the nature of the bleeding (acute vs. chronic), amount and duration of flow, and based on the clinical presentation a targeted clinical assessment based on age. The basic structure of the suggested investigations includes an assessment of the duration of flow, a measurement of hemoglobin and/or hematocrit, a pelvic ultrasound, an assessment of the endometrial cavity, and at least a structured medical history to assess for possible coagulopathy ( Table 35.5 ).
| Initial screening for an underlying disorder of hemostasis in patients with excessive menstrual bleeding should be structured by the medical history. A positive screening result comprises the following circumstances: |
| Heavy menstrual bleeding since menarche |
| One of the following: |
| a. Postpartum hemorrhage |
| b. Surgical related bleeding |
| c. Bleeding associated with dental work |
| Two or more of the following conditions: |
| a. Bruising, 1–2 times/month |
| b. Epistaxis, 1–2 times/month |
| c. Frequent gum bleeding |
| d. Family history of bleeding symptoms |
| Patients with a positive screening result should be considered for further evaluation, including consultation with a hematologist and testing for von Willebrand factor, ristocetin cofactor. |
Acute Abnormal Uterine Bleeding
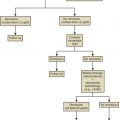
Acute AUB may range in severity from excessive menstrual bleeding to hemorrhage resulting in hypovolemic shock. In the unstable patient, management should be directed at establishing the appropriate intravenous (IV) lines and obtaining cross-matched blood to correct conditions of hypovolemia and anemia. These steps are performed simultaneously with obtaining enough of a history and a physical exam to confirm the bleeding is indeed arising from the uterus, and to exclude pregnancy in women of reproductive age. Appropriate resuscitation is the priority, and procedural management may be necessary ( Fig. 35.21 ).
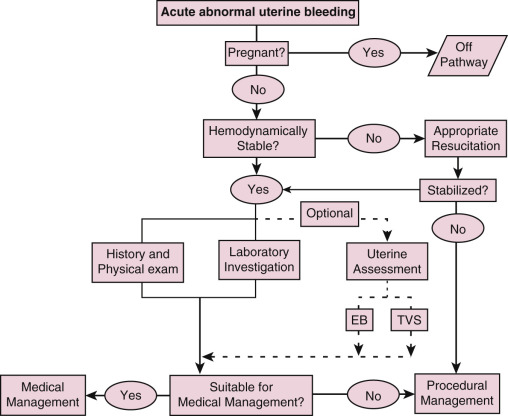
In a stabilized patient, screening questions should be focused to elicit a possible personal or family history of congenital or acquired coagulopathy (see Table 35.5 ). Additional laboratory testing ( Table 35.6 ) may be indicated when the structured history questions for coagulopathy are positive, and vWD should be considered, especially in adolescents presenting with acute AUB. Serum testing for coagulation factors may be affected by the time in the menstrual cycle or the use of gonadal steroids, and it may bear repeating if the patient history is suggestive. For women who are on therapeutic anticoagulants, an international normalized ratio and measures of coagulation function should be assessed. A pelvic exam may elicit the potential etiologies such as pregnancy, a prolapsing myoma, or adenomyosis. A history of a recent uterine curettage for the treatment of miscarriage or termination of pregnancy, or a history of gestational trophoblastic disease, may alert the physician to a possible uterine AVM. AVM, which may be congenital or acquired, while considered rare, may lead to severe life-threatening bleeding. The ultrasonographic diagnosis of AVM is based on the presence of hypoechoic tortuous spaces within the myometrium demonstrating vascular flow as evidenced by color Doppler.
| Laboratory Evaluation | Specific Laboratory Tests |
|---|---|
| Initial laboratory testing | Complete blood count with platelets Blood type and cross match Pregnancy test, urinary or serum |
| If suspicion for systemic coagulation disorder | Partial thromboplastin time (PTT) Prothrombin time Activated partial thromboplastin time (aPTT) Fibrinogen level International normalized ratio (INR) Plasma sample for storage |
| If at risk for inherited coagulopathy or “positive” structured history Initial laboratory testing for von Willebrand disease | von Willebrand factor antigen Ristocetin cofactor assay Factor VIII |
| Ultrasound | Transvaginal ultrasound |
| Other | Thyroid-stimulating hormone Serum iron, total iron binding capacity, and ferritin Liver function tests Endometrial sampling Full hemostatic evaluation as personal or family history suggests Chlamydia trachomatis |
Depending on the feasibility of achieving a meaningful specimen, and the clinical indications, the provider may choose to perform an endometrial sampling. Cytologic assessment of the cervix, histological evaluation of the endometrium, and sonographic and hysteroscopic evaluation of the structure of the endometrial cavity may be limited secondary to the amount of bleeding and the presence of intrauterine blood clot. In the presence of hemodynamic instability, these measures may be deferred to take appropriate medical or procedural intervention. Endometrial sampling is not recommended in all women with acute heavy bleeding, but in women over the age of 45, and those who are at increased risk for EIN or cancer (chronic anovulation, diabetes, obesity, a family history of EC, prolonged exposure or unopposed estrogen use, or tamoxifen use), outpatient endometrial sampling, or hysteroscopy, D&C should be performed.
Ultrasound Imaging of Endometrial Cavity in Acute Abnormal Uterine Bleeding
In the nonpregnant women with acute AUB, a TVS that demonstrates a thin endometrial lining in combination with an absence of leiomyomata near the endometrial echo has been associated with normal results at hysteroscopic assessment of the uterine cavity. In the setting of acute AUB, TVS is a suitable screening tool, but the results may be hindered by echogenic clot or debris with mixed echogenicity within the endometrium. Routine TVS is less sensitive than an SHG (SIS) or hysteroscopy in the detection of intrauterine lesions such as polyps; thus once the vaginal bleeding has remitted, a further assessment of the detail of the anatomy of the uterine cavity may be warranted.
Hormonal Treatment of Acute Abnormal Uterine Bleeding
Once the patient has been resuscitated, a variety of treatment options are available to diminish bleeding ( Tables 35.7 and 35.8 ). Once the acute bleeding has slowed, these regimens are tapered to a maintenance dose. TA is a synthetic derivative of the amino acid lysine and exerts its antifibrinolytics effect through the reversible blockade of lysine-binding sites on plasminogen molecules. Both IV and oral formulations are available. IV conjugated equine estrogen is effective in treating acute AUB and is generally used as 25 mg IV every 4 hours up to 24 hours to obtain the cessation of bleeding. Patients should then be transitioned to a regimen of monophasic multidose oral contraceptives (OCs), or to moderate- or high-dose progestin regimens. Monophasic, combination OCs have been used successfully in the treatment of acute AUB, with the cessation of bleeding on average 3 days after the initiation of therapy. An alternative to the combination OCs, and reported to be equally effective, is a regimen of medroxyprogesterone acetate 20 mg administered three times a day for a week, followed by a gradual taper.
| First Line |
|
| Second Line (Dependent on Desire for Future Fertility) |
|
| Maintenance Therapy |
|
| Medication | Route | Protocol for Acute Menstrual Cessation | Contraindications |
|---|---|---|---|
| Tranexamic acid | IV Oral | 10 mg/kg Q8 h 20–25 mg/kg Q8 h 1.3 g TID * | Disseminated intravascular coagulation, venous or arterial thromboembolism, macroscopic hematuria, color blindness |
| Conjugated estrogen | IV | 25 mg Q 4–6 h until bleeding stops, re-evaluated need at 48 h | Pregnant, active or previous venous or arterial thromboembolic disease, breast cancer, use with caution in obese women |
| 30 µg ethinyl estradiol/0.3 mg norgestrel (or other 30 or 35 µg combination pill) | Oral | One tablet Q 6 h until bleeding stops, re-evaluate at 48 hours, then taper dose over several weeks | Pregnant, smoking (aged ≥35 years and ≥15 cigarettes/day), history of malabsorptive bariatric surgery, multiple risk factors for arterial cardiovascular disease, hypertension, active or previous venous or arterial thromboembolic disease, known thrombogenic mutations, current or past heart disease, systemic lupus erythematosus with vascular disease, nephritis, or antiphospholipid antibodies, headache with aura, current or past history of breast cancer, diabetic neuropathy, retinopathy, nephropathy, or diabetes ≥20 years, liver cirrhosis, or tumor |
| 50 µg ethinyl estradiol/0.5 mg norgestrel (or other 50 µg combination pill) | Oral | One tablet Q 6 h until bleeding stops, re-evaluate at 48 hours, then taper dose over several weeks | Same as 30 to 30 µg pill |
| Norethindrone acetate | Oral | 20 mg three times a day for 7 days, may taper as bleeding stops, taper over several weeks | Pregnant, history of malabsorptive bariatric surgery, liver disease or tumor, breast cancer, current or past ischemic heart disease |
| Medroxyprogesterone | Oral | 10 mg Q 4 h (up to 80 mg/day) until bleeding stops then taper over several weeks | Same as norethindrone acetate |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree