Stomach Cancer
 ANATOMY
ANATOMY
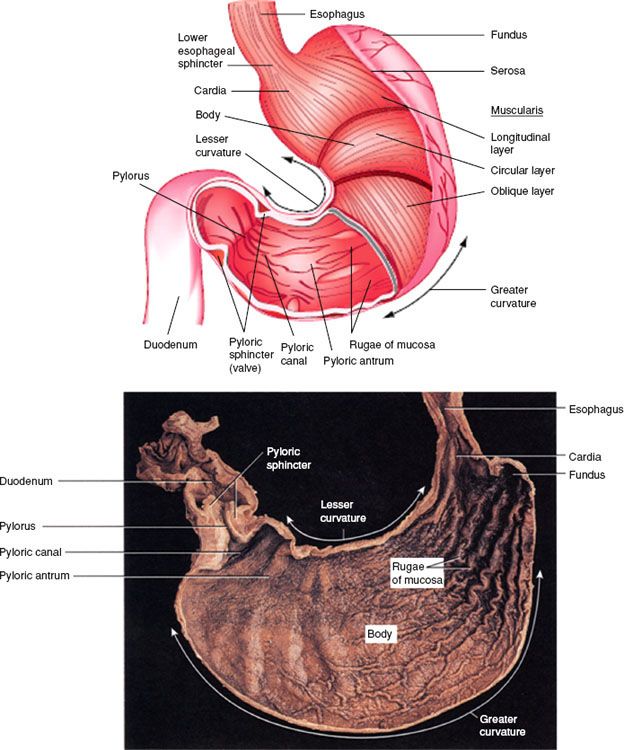
The stomach begins at the gastroesophageal (GE) junction and ends at the pylorus. The stomach is generally divided into anatomic regions, including the gastric cardia (the region surrounding the superior opening of the stomach where it connects with the GE junction/level of the lower esophageal sphincter), the fundus (situated superiorly, a rounded portion superior to the body and to the left of the cardia), the body (a large, central portion of the stomach), and the pyloric canal (distally, connecting to the duodenum). The pylorus is composed of two parts: the pyloric antrum, which connects to the body of the stomach, and the pyloric canal, which empties into the duodenum (Fig. 58.1). The pylorus communicates with the duodenum of the small intestine via the pyloric sphincter (valve). This valve regulates the passage of chyme from stomach to duodenum and prevents backflow of chyme from duodenum to stomach. A plane passing through the incisura angularis on the lesser curvature divides the stomach into the body and the pyloric portion (antrum). The anterior surface of the stomach is covered with peritoneum of the greater sac. At the left and cranially, it abuts the diaphragm. In view of the increasing incidence of gastric cancer at the GE junction, it is important to note that there is either no or variable visceral peritoneal covering at the most proximal portion of the GE junction.1 Positive radial margins at this site are often “true” positive margins, whereas many other positive margins in the stomach are free serosal margins unless the tumor is adherent to an adjacent organ or structure. The right portion of the anterior gastric surface is adjacent to the left lobe of the liver and the anterior abdominal wall. Posteriorly, the stomach is covered with peritoneum of the lesser sac or omental bursa. The stomach contacts many visceral structures; from superior to inferior, it is adjacent to the spleen, left adrenal gland, superior portion of the left kidney, ventral portion of the pancreas, and transverse colon. The hepatogastric ligament or lesser omentum is attached to the lesser curvature and contains the left gastric artery and the right gastric branch of the hepatic artery. Histologically, the wall of the stomach has five layers: the mucosa, the submucosa, muscular layer, subserosa, and serosa. The muscularis layer is composed of an outer longitudinal layer, a middle circular layer, and inner oblique layer.
The stomach’s vascular supply is derived from the celiac axis. The celiac artery usually has three branches: the left gastric artery, which supplies the upper right portion of the stomach; the common hepatic artery, which gives rise to the right gastric artery supplying the lower right portion of the stomach, and the right gastroepiploic branch supplying the lower portion of the greater curvature; and the splenic artery, which gives rise to the left gastroepiploic supplying the upper portion of the greater curvature and the short gastric arteries supplying the fundus. Variations in this normal vascular supply are common. The celiac axis originates at or below the pedicle of T12 in approximately 75% of patients and at or above the pedicle of L1 in 25% of patients.2 The lymphatic drainage of the stomach follows the arterial supply. Although most lymphatics ultimately drain to the celiac nodal basin, lymph drainage sites can include the splenic hilum, suprapancreatic nodal groups, porta hepatis, and gastroduodenal areas.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Gastric cancer is estimated to afflict 21,320 people in the United States in 2012 and result in 10,540 deaths.3 Worldwide, gastric cancer remains a significant cause of cancer-related mortality, with nearly one million new cases annually and an estimated 700,000 deaths, making it the second-leading cause of cancer-related deaths. During the past 70 years, there has been a significant decline in the incidence of gastric cancer in both sexes in Western countries. The causes of this decline are unknown.4,5 Although the overall decrease in gastric cancer incidence is encouraging, there has been a steady and dramatic increase in the incidence of proximal lesser curvature, cardia, and GE junction tumors over the past 20 years, especially in White males.6 In contrast to the increasing incidence of more proximal gastric cancers seen in the Western Hemisphere and parts of Europe, distal tumors continue to comprise most gastric cancers in the Far East and other parts of the world.
Risk factors implicated in gastric cancer development include consumption of smoked, pickled, and salted foods; low intake of fruits and vegetables; low socioeconomic status; smoking; decreased use of refrigeration; and dried meat/fish consumption.4,7 Pernicious anemia is associated with gastric cancer, with 5% to 10% of patients with pernicious anemia developing gastric cancer. Prior subtotal gastrectomy for benign lesions also carries a 25% risk for subsequent malignancies, with latency periods of 15 to 40 years.8 Villous adenomas are clearly premalignant; hyperplastic or hamartomatous polyps occur more frequently and are apparently benign. Gastric ulcers carry no increased risk, although previous distal gastrectomy for benign disease confers a 1.5- to 3-fold relative risk for development of gastric cancer, with a latency period of 15 to 20 years. Additionally, patients with a family history of nonhereditary gastric cancer have a higher risk for developing similar disease, with a very small percentage of patients developing gastric cancer in the context of an inherited syndrome. The presence of Helicobacter pylori is associated with a three to six times greater risk of gastric cancer than if infection is absent. The increased association of H. pylori appears to be confined to those with distal gastric cancer and intestinal-type malignancy.9 Although this association of H. pylori infection with gastric cancer may provide new insight into the pathogenesis of this tumor, only a small minority of infected people develop gastric carcinoma, and there are no firmly established data regarding the screening of infected patients or the effect of treatment for infection on subsequent malignancy.
Historically, gastric adenocarcinomas have been diagnosed as intestinal or diffuse types. Intestinal types are more prevalent in high-incidence areas and responsible for much of the observed global ethnic variation of disease.10 In contrast, diffuse gastric cancer incidence is fairly uniform among geographic regions or race and more uncommon than intestinal type. More recently, there has been an emergence of another distinct subtype—proximal gastric cancers. These are often grouped with GE junction and distal esophageal adenocarcinomas, with a rapidly rising incidence in industrialized nations. The risks factors for these individual types appear to vary significantly. Risk factors of intestinal, noncardia gastric cancers appear to center around inflammatory causes and environmental factors, including H. pylori, chronic gastritis, tobacco, high salt intake, and alcohol consumption. The less common diffuse gastric cancer subtype appears to be distinct, with E-cadherin mutations/silencing thought to be a common precursor event with this histology. Hereditary diffuse gastric cancer is a genetic syndrome in which there is loss of function/mutation in the E-cadherin gene. In proximal gastric cancer (and in contrast to intestinal types), H. pylori infection has been found to be potentially protective, possibly through associated atrophic gastritis and reduced acid production with subsequent reduction in gastroesophageal reflux disease (GERD). This possible association remains an ongoing area of study.10
FIGURE 58.1. Gastric anatomy. (From Tortora GJ, Grabowski SR. Principles of anatomy and physiology, 9th ed. New York: John Wiley & Sons, Inc. Copyright 1996. Reprinted with permission of John Wiley & Sons, Inc.)
 PATTERNS OF SPREAD
PATTERNS OF SPREAD
Cancer of the stomach may extend directly into the omenta, pancreas, diaphragm, transverse colon or mesocolon, and duodenum. Peritoneal contamination with carcinomatosis is possible after a lesion extends beyond the gastric wall to a free peritoneal (serosal) surface.11
Microscopic or subclinical spread beyond the visible gross lesion occurs frequently because of the abundant lymphatic channels within the submucosal and subserosal layers of the gastric wall. The submucosal plexus in the esophagus and subserosal plexus in the duodenum allow proximal and distal spread.
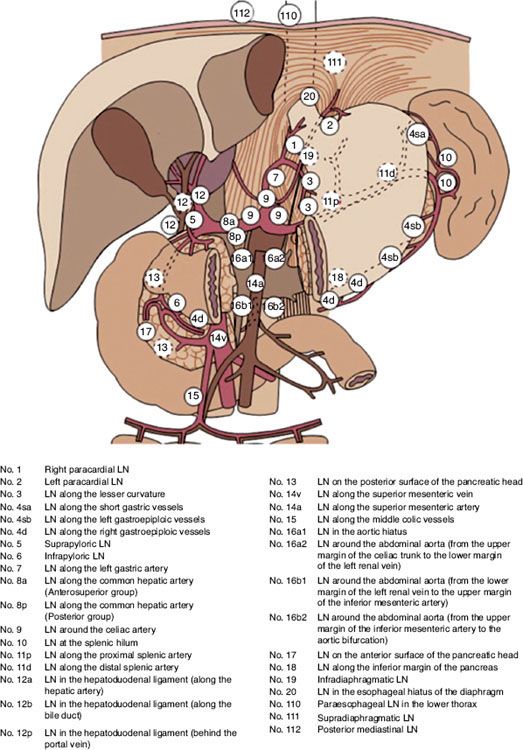
It is difficult to perform a complete node dissection because of the numerous pathways of lymphatic drainage from the stomach (Fig. 58.2). Initial drainage is to lymph nodes along the lesser and greater curvatures (i.e., gastric and gastroepiploic nodes) but also includes the celiac axis, porta hepatis, splenic, suprapancreatic, pancreaticoduodenal, adjacent para-aortics, and distal paraesophageal system. The relative risks for nodal involvement in gastric cancer depend on the location of the primary tumor as well as extent of gastric wall involvement.
Gastric venous drainage is primarily to the liver by the portal system. Liver involvement is found in as many as 30% of patients at initial exploration, and it can occur as a result of venous metastasis, peritoneal-based spread, and direct extension (Fig. 58.3).
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
The most common presenting symptoms of stomach cancer are loss of appetite, early satiety, abdominal discomfort, unintentional weight loss, anemia-related weakness, nausea and vomiting, and tarry stools. Duration of symptoms is <3 months in almost 40% of patients and >1 year in 20%. Physical examination can reveal advanced disease, for which the presentation may include an abdominal mass (epigastric or liver mass as well as a periumbilical node [i.e., Sister Mary Joseph node]), palpable left supraclavicular nodes (i.e., Virchow’s node), or a rectal shelf (representing peritoneal seeding [i.e., Blumer’s shelf]).
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
Diagnosis is usually confirmed by upper gastrointestinal endoscopy as well as imaging studies in some instances. Double-contrast x-ray studies may reveal small lesions limited to the inner layers of the gastric wall. Endoscopy with direct visualization, cytology, and biopsy yields the diagnosis in ≥90% of exophytic lesions; however, infiltrative (linitis plastica), small (<3 cm), or cardia lesions may be more difficult to diagnose endoscopically. Endoscopic ultrasonography is the most accurate method of determining depth of tumor invasion (intramural vs. extramural extension) prior to resection but is less accurate in detecting regional nodal metastases.12,13 In some institutions, needle biopsies of suspicious nodes are performed at the time of endoscopic ultrasound.
Abdominal computed tomography (CT) is useful in determining the abdominal extent of disease and may help to determine which lesions extend to surgically unresectable structures but are of lesser value in detecting small lymph node metastases or peritoneal spread. Distant metastases should be ruled out with contrast-enhanced CT of the chest, abdomen, and pelvis. CT may provide valuable tumor localization information if irradiation is indicated. If a proximal gastric cancer extends to involve the esophagus, CT of the chest will help to rule out involvement of mediastinal nodes or the lung parenchyma.
Helical CT may be more useful than conventional CT in identifying smaller lymph nodes, which are particularly relevant to staging of gastric cancer patients. In a single-institution experience, 58 patients underwent nodal dissection for gastric cancer.14 A total of 1,082 nodes were resected, and 138 were metastatic. Helical CT was able to identify 1.1% of the 649 lymph nodes that were 1 to 4 mm in diameter, 45.1% of the 355 nodes of 5 to 9 mm, and 72% of the nodes >9 mm. For nodes ≥5 mm, the sensitivity for identifying metastatic nodes was greater than for nonmetastatic nodes (75.2% vs. 41.8%, respectively).
The value of laparoscopy in the staging of gastric cancer remains a subject of study. In one study of 71 patients with CT criteria of resectable disease, 69 completed laparoscopic evaluation.15 Forty-one of the 69 patients proceeded to laparotomy with curative intent, and 38 of 41 patients had resection of all gross tumor. Pathologic evidence documented hepatic metastasis in three patients with a negative CT scan of the liver, and one of them was detected by laparoscopy. Laparoscopy confirmed disease in 16 of 17 patients with peritoneal metastases (avoiding 12 laparotomies, 17%). The combination of CT scan and laparoscopy for staging information yielded a resectability rate of 93% in patients defined as potential candidates for curative gastric cancer surgery. Similarly, a large series from Memorial Sloan-Kettering Cancer Center of >600 patients with potentially resectable gastric adenocarcinoma by conventional imaging underwent laparoscopic staging, resulting in distant metastases being detected in 31% of patients.16 A systematic review on the role of diagnostic laparoscopy showed that change in patient management occurred in 9% to 60% of patients initially deemed resectable by preoperative imaging, predominantly through the identification of patients with metastatic disease who would not benefit from either laparotomy or neoadjuvant therapy, thereby avoiding unnecessary interventions. This primarily related to patients with advanced (T3, T4) gastric cancer, with relatively minimal benefit in early-stage patients, prompting the authors to recommend diagnostic laparoscopy for patients with locally advanced-stage disease.17 In addition, peritoneal fluid can be sampled for cytology at laparoscopy. If positive, this is a poor prognostic indicator and the patient should be considered as having M1 disease.
Overall treatment planning in gastric cancer may be improved by accurate preoperative classification of key prognostic factors, namely depth of invasion (T category) and lymph node involvement (N category). A prospective study in 108 patients evaluated endogastric ultrasonography (EUS), CT, and intraoperative surgical assessment for T and N classification.18 T-staging was accurately characterized by CT in 43% of the cases, EUS in 86% of cases, and intraoperative assessment in 56% of cases. Staging of N1 and N2 lymph nodes was correct with CT in 51% of the cases, 74% with EUS, and 54% with intraoperative assessment. Advanced gastric tumors tended to be more accurately staged with CT, although CT, in general, overstages the T-category and understages the N category. EUS showed a high accuracy for all the T-categories, although it also tends to understage N-categories. Finally, intraoperative assessment was equally accurate for all N-categories but tended to overstage early T-stages and to understage N-categories. A meta-analysis and systematic review of the role of EUS in T- and N-staging in nearly 2,000 patients with gastric cancers suggested the sensitivity for detecting T1, 2, 3, and 4 disease was 88, 82, 90, and 99%, respectively, with nodal staging sensitivity for N1 and N2 disease 58 and 65%, respectively, also suggesting that accuracy was higher with more advanced disease.19 Another systematic review concluded that EUS, multidetector CT, and MRI achieve similar results in terms of diagnostic accuracy of T-staging and assessing serosal involvement. Given that most experience has been gained with EUS, this was felt to remain as the first-choice imaging modality in the preoperative T-staging of gastric cancer.20 However, it should be remembered that EUS accuracy highly depends on the experience and expertise of the operating physician.
FIGURE 58.2. Locations of gastric lymph node stations. (From Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol 2009;92:164–175, with permission from Elsevier.)
The positron emission tomography (PET) scan has a lower detection rate in some cases of gastric cancers owing to the low fluorine-18 fluorodeoxyglucose (FDG) accumulation in patients with diffuse or mucinous tumors.21 Approximately 40% of gastric carcinomas, notably above histologies, may not be detected with PET scan.22 PET alone has been described as displaying a lower sensitivity when compared to CT in detecting nodal involvement, although specificity is improved.23 Given this, PET alone is likely not an adequate diagnostic procedure for evaluation of gastric cancer and generally should be used in conjunction with CT scan for correlation, allowing more accurate preoperative staging than either modality alone. A suggested diagnostic evaluation for gastric cancer is shown in Table 58.1.
A prospective report analyzed the potential involvement of bone marrow at the time of radical surgery after obtaining material through aspiration and the use of monoclonal antibody CK-2 directed to component 18 of the intracellular cytokeratin.24 Among the 180 patients evaluated, 53% had a positive test showing malignant cells in the bone marrow. The finding was correlated with pT (p = .07) and Borrmann’s classification (p = .02) (see later discussion).25 The estimation of tumor cell contents in the bone marrow (<3 é 106) was related significantly to overall and disease-free survival (p = .04 and p <.007, respectively). The multivariate analyses detected that bone marrow malignant involvement was an independent prognostic factor for disease-free survival in pT1 to pT2 stages (p = .004), intestinal histologic subtypes (p <.008), and N0 patients (p = .004).
FIGURE 58.3. Patterns of failure in 82 evaluable patients in the University of Minnesota Reoperation series. A: Large bold circles indicate local failures in surrounding organs or tissues; large open circles indicate lymph node failures. B: Asterisk (*) indicates lung metastasis; plus (+) indicates liver metastasis. Superimposed irradiation portals encompass postsurgical gastric remnant, anastomoses, duodenal stump, gastric bed structures, and primary and secondary areas of lymph node drainage; broken lines represent upper and total abdomen fields. (From Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a reoperation series [second or symptomatic looks]. Clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1, with permission from Elsevier.)
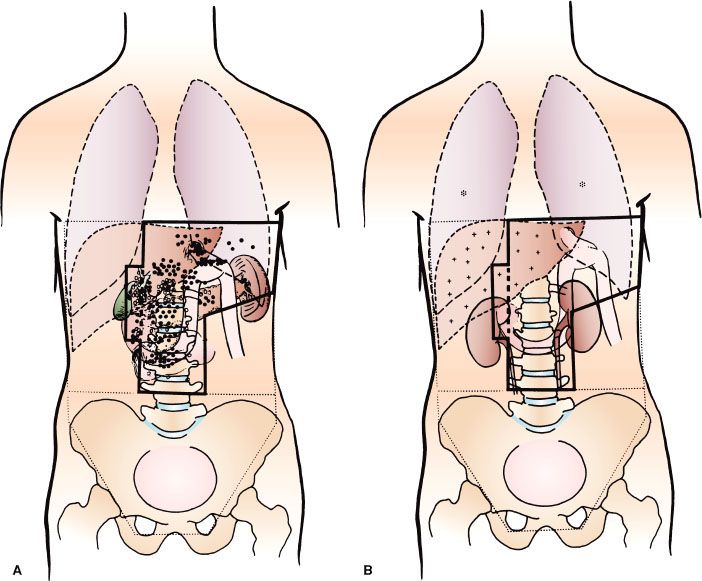
TABLE 58.1 DIAGNOSTIC WORKUP FOR GASTRIC CANCER

 STAGING
STAGING
The current tumor-node-metastasis (TNM) system is depicted in Table 58.2.26 In the most recent American Joint Committee on Cancer (AJCC) staging of gastric cancers, cancers whose midpoint is in the lower thoracic esophagus, GE junction, or within the proximal 5 cm of the stomach (cardia) and extending to the GE junction or esophagus are staged as esophageal neoplasms. All other cancers with a midpoint in the stomach lying more than 5 cm distal to the GE junction or those within 5 cm of the GE junction (but not involving the GE junction or esophagus) are staged using the gastric cancer staging system. Potential drawbacks of this system are that it is based primarily on surgical outcomes and not entirely suitable in considering clinical baseline staging and preoperative therapy.
TABLE 58.2 AMERICAN JOINT COMMITTEE ON CANCER 2010 GASTRIC CANCER STAGING
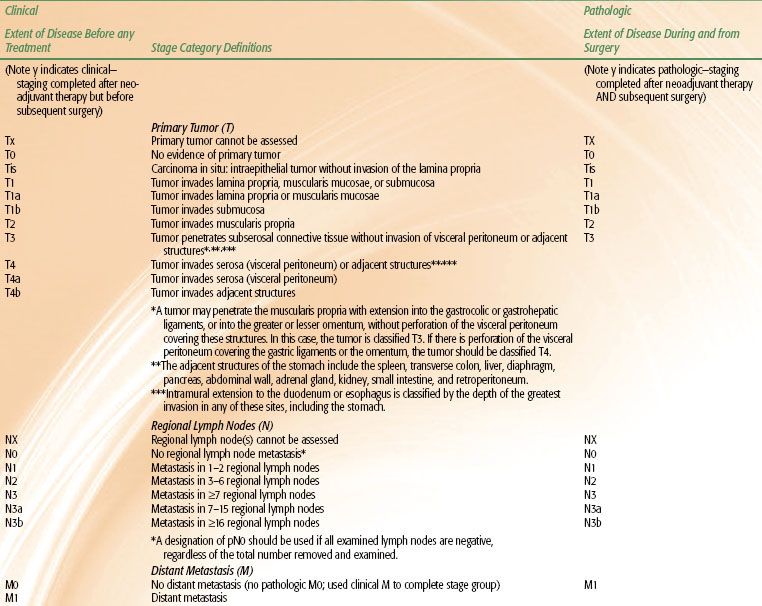
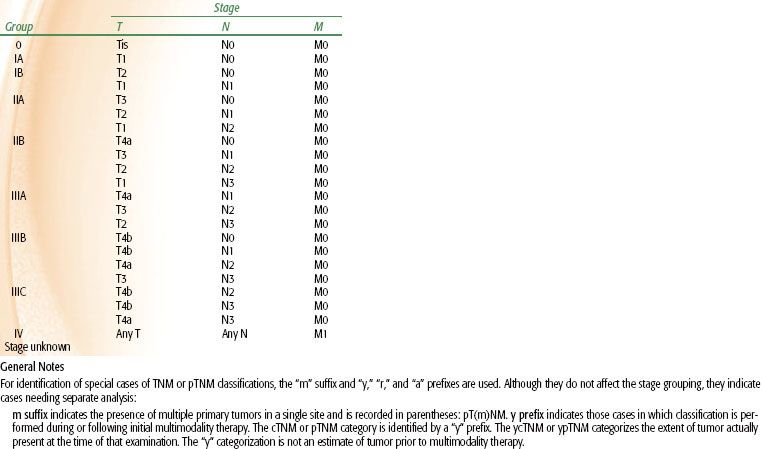

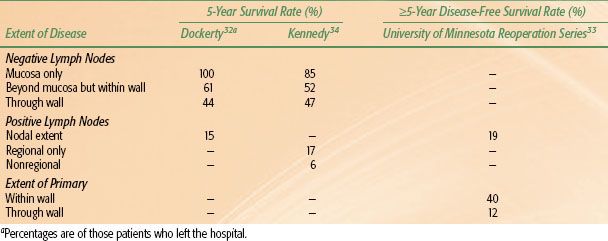
TABLE 58.3 EXTENT OF INITIAL DISEASE COMPARED WITH SURVIVAL RATES FOR CANCER OF THE STOMACH
 PATHOLOGY
PATHOLOGY
Adenocarcinomas account for 90% to 95% of all gastric malignancies. Lymphomas, including both favorable and unfavorable histologies, are the second most common malignancies. Rarely, leiomyosarcomas (2%), carcinoid tumors (1%), adenoacanthomas (1%), and squamous cell carcinomas (1%) occur.
The site of origin of gastric cancers within the stomach has changed in the United States over recent decades, and proximal lesions are being diagnosed and treated more frequently. Although the highest frequency is still in the antrum/distal stomach (approximately 40%), the lowest frequency is now in the body rather than proximal portion of the stomach (approximately 25%), with intermediate frequency in the proximal stomach and GE junction (approximately 35%). Several investigators have reported an increased frequency of cardia lesions. As described previously, cardia lesions appear to have different epidemiologic factors, exhibit different tumor biology, and have an inferior prognosis from lesions in the other sites.27–28,29–31 Gastric cancers sometimes are categorized according to Borrmann’s five types. Type I tumors are polypoid or fungating; type II are ulcerating lesions surrounded by elevated borders; type III have ulceration with invasion of the gastric wall; type IV are diffusely infiltrating (linitis plastica); and type V are unclassifiable.25
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
The most important prognostic indicators reflect tumor extent. If hematogenous or transperitoneal spread is present, the outcome is, essentially, uniformly fatal. Survival rates decrease with progressive tumor extension within or beyond the gastric wall.27,32,33,34 The number of involved lymph nodes also has a significant impact on survival. Lymph node involvement is important, as are the number and locations of nodes affected.27,34,35,36 Minimal node involvement adjacent to the primary lesion only moderately affects prognosis.32,36,37 The finding of either involved lymph nodes or complete wall penetration is usually not as ominous as the presence of both32,34,36,38 (Table 58.3). Additional prognostic indicators include a poor performance status, elevated alkaline phosphatase levels, and ethnicity.39 In one study, Asian-Pacific Islanders born in the United States were compared to those born abroad, finding that only foreign-born Asian-Pacific Islanders had a more favorable survival compared to indigenous Caucasians.40
Flow cytometry is also prognostically valuable; aneuploidy is associated with unfavorable tumor location, lymph node metastasis, and primary tumor invasion.41–43 Unfavorable DNA flow cytometry correlates with a poor prognosis.41 The prognosis is worse for cardia lesions, and flow cytometry reveals a greater incidence of aneuploidy.43 The gross pathologic appearance of the primary lesion also reveals prognostic information, although it is not known whether this factor is independent of tumor stage. Patients with Borrmann type I and II tumors have relatively favorable 5-year survival rates, although patients with type IV (linitis plastica) fare poorly.37,44–46
The molecular biology of gastric cancer reflects the heterogeneity of its causes and its histologic subtypes. Identification of the genetic and phenotypic variables existing among gastric cancers may lead to more directed therapeutic approaches and a more accurate prediction of clinical outcome. Changes that may affect the behavior of gastric tumor cells involve four major types of alterations. Loss of tumor suppressor gene function, especially inactivation of the p53 gene, appears to play a critical role. The p53 gene is located on the short arm of chromosome 17 and plays a key role in tumor suppression and cell-cycle regulation.47 The p53 gene halts DNA replication and triggers programmed cell death in response to DNA damage.48 Loss of p53 function allows malignancy to develop, affects the effectiveness of chemotherapy and irradiation, and predisposes cells to genetic instability.49,50 The latter is particularly important because p53 mutations occur early in tumorogenesis.51
A second major aberration affecting gastric epithelial cells is the impact of alterations in mismatch repair genes. Two such genes, hMSH3 and hMLH1, on chromosome 2 and 3, respectively, account for replication errors throughout the genome. Mutations in these genes are implicated in cancer family syndromes and hereditary nonpolyposis colorectal cancer, which is a disease associated with an increased tendency for the development of gastric tumors.52 Mutations in these genes generate genetic instability and have the potential to lead to further alterations in oncogenes.
Two proto-oncogenes, c-met and k-sam, are associated with scirrhous carcinoma of the stomach. The former encodes hepatocyte growth factor, which is a potent endogenous promoter of gastric epithelial cell growth.53 Its overexpression correlates with tumor progression and metastasis.54 The latter encodes a tyrosine kinase receptor family.54 In scirrhous carcinoma, c-met and k-sam amplification may occur independently. There is a tendency for k-sam to be activated in women <40 years of age and c-met to be amplified in men >50 years of age.51,55
Peptide receptors, including estrogen receptors and epidermal growth factor receptors, are associated with adverse prognoses.56,57 Epidermal growth factor receptors and levels correlate with higher rates of primary tumor infiltration, poorer histologic differentiation, and linitis plastica.58 The pathophysiologic relation between these peptide receptors and their association with poor prognoses is not well understood.
Modern molecular biology observations confirm the heterogeneity of human gastric cancer. Genetic alterations detected and potentially associated with a worse prognosis include CD44 expression; telomerase reactivation; p53 gene inactivation; dysfunction of repair genes such as hMSH3 and hMLH1; overexpression of proto-oncogenes such as erb-B2, bcl-2, c-met, and k-sam; estrogenic receptor expression; and presence of viral genomes.59 Gastric cancers with class II major histocompatibility complex antigen expression have a better prognosis; however, the loss of expression is not an independent prognostic factor.58 Illustrating the importance of understanding and potential exploitation of these genetic alterations, a recent randomized study was conducted in patients with HER2 overexpression. Patients with locally advanced or metastatic gastric or GE cancer (the ToGA trial) were randomized to determine whether trastuzumab (an antibody against the HER2 gene product/receptor) enhanced treatment efficacy when added to cisplatin and 5-fluorouracil (5-FU)/capecitabine therapy. This study showed that the addition of trastuzumab achieved a significant improvement in overall and progression-free survival.59a These and other data have led to the initiation of the Radiation Therapy Oncology Group (RTOG) 1010 trial, which randomized patients with esophageal/GE junction tumors to receive preoperative radiation therapy concurrent with paclitaxel/carboplatin, with or without trastuzumab, followed by resection.
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
Surgical Management
Operative attempts are highly successful if disease is limited to the mucosa; however, the incidence of such early lesions at diagnosis is <5% in most U.S. series. In Japan, the incidence of lesions initially confined to the mucosa or submucosa was only 3.8% in 1955 and 1956, although by 1966, as a result of screening procedures, this figure had increased to 34.5%, with corresponding survival rates of 90.9%.60 For very early gastric cancer, endoscopic mucosal resection and endoscopic submucosal dissection have been successfully used as less radical alternatives to standard surgery. Endoscopic laser surgery has been applied successfully to patients with very early gastric cancer whose tumors are inoperable because of complicating medical illness. Small lesions that are pedunculated, noninvasive, and well differentiated have lymph node metastasis in <5% of cases and can be completely removed endoscopically in 75% of cases.61 Radiation therapy with chemotherapy may be considered as adjuvant therapy in selected situations. These approaches in early gastric cancer (uncommonly seen in Western society) require meticulous patient selection and remain a topic of investigation.
Curative or palliative surgical resection is possible for 50% to 60% of patients at the time of initial disease presentation. However, only approximately 25% to 40% are eligible for potentially curative resection. Generally, patients with evidence of peritoneal involvement, distant metastases, or locally advanced disease (including encasement of unresectable/major blood vessels) are generally considered to have unresectable disease. Palliative resection is usually reserved for rare cases, including symptomatic palliation of bleeding uncontrolled by other methods. In some instances, unresectable tumors may be debulked successfully, with sites of minimal residual disease marked judiciously with clips. This may palliate and permit accurate delivery of postoperative radiation therapy. In patients with gastric outlet obstruction, gastrojejunostomy may be performed and preferable to endoscopic placement of stents in patients with expected longer survival.
No prospective randomized trials have definitively established optimal surgical therapy.62 Generally, it is recommended that gastric cancer surgery be performed by experienced surgeons in high-volume centers, entailing removal of the perigastric lymph nodes (D1) as well as those along the main vessels of the celiac trunk (D2), with the goal of examining ≥15 lymph nodes (discussed later).6 The preferred treatment for gastric carcinoma, especially for lesions arising in the body and antrum, is a radical subtotal gastrectomy. This operation removes approximately 80% of the stomach along with the node-bearing tissue, the gastrohepatic and gastrocolic omenta, and the first portion of the duodenum. Larger lesions may require total gastrectomy. For more proximal tumors, both proximal and total gastrectomy may be appropriate depending on extent of disease. There appears to be no advantage to performing total gastrectomy if subtotal gastrectomy produces satisfactory margins (i.e., 5 cm). Patients treated with total gastrectomy characteristically have 5-year survival rates of 10% to 15%, and those undergoing radical subtotal gastrectomy have 5-year survival rates of 25% to 45%.35,63,64 The inferior survivorship of patients undergoing total gastrectomy probably reflects larger tumors and unfavorable proximal lesions that prompt such a procedure. The value of splenectomy has not been addressed in prospective randomized trials; however, retrospective Japanese data do not support a survival benefit.47,65 Because routine splenectomy has not shown significant improved outcomes and potentially increases complication rates, complete removal of splenic nodes is not commonly performed by Western surgeons. The use of laparoscopic approaches in gastric cancer resection may reduce blood loss while potentially decreasing length of postoperative hospital stay and remains the subject of ongoing investigation. In patients who undergo adjuvant chemoradiotherapy, it may be prudent to place feeding jejunostomy at the time of resection.
The propensity for gastric carcinoma to spread via the submucosal lymphatics suggests that a 5-cm margin of normal tissue proximally and distally may be optimal. It may be necessary to include a portion of esophagus or duodenum to achieve adequate margins. Frozen-section pathologic evaluation of surgical margins has been advocated to confirm their adequacy.35 The importance of careful evaluation of longitudinal margins is emphasized in several series (Table 58.4) with documented positive pathologic margins in approximately one-quarter of “curatively” resected specimens.66–68,69,70–73 The approximate 25% positive longitudinal margin correlates almost precisely with the incidence of locoregional recurrence in the anastomosis or stump, as discussed later in this chapter.
Although R0 resection with adequate lymph node sampling is a primary goal for gastric cancer surgery, approximately half of patients end up with uninvolved margins. Although the longitudinal margin is routinely evaluated, equally important but frequently not assessed are the radial or circumferential margins. The incidence of radial margin positivity is not well reported in the literature (most of the series referenced in Table 58.4 addressed longitudinal margins alone). The rising incidence of T3 and T4 GE tumors will likely result in an increasing rate of microscopically positive radial margins. Because the perigastric tissue surrounding the GE junction and distal esophagus has no serosa, lesions that extend to the pathologic radial margin represent a true positive margin in a large percentage of cases.
The extent of lymph node dissection is controversial. At resection, it is recommended at least 15 lymph nodes be retrieved to reduce stage migration. In a D0 dissection, there is generally incomplete removal of the lymph nodes along the greater and lesser curvature. D1 dissection refers to removal of nodes along the lesser curvature (nodal stations 1, 3, and 5) and greater curvature (nodal stations 2, 4, and 6) (Fig. 58.2). In addition, more extensive lymph node dissection, including removal of nodes along the left gastric artery (nodal station 7), common hepatic artery (nodal station 8), celiac trunk (nodal station 9), and splenic artery (nodal stations 10 and 11) are referred to as a D2 dissection. In series with rigorous pathologic evaluation of these nodes,74 the likelihood of discovering lymph node metastasis increases markedly in both D1 and D2 procedures. There appears to be a small subset of patients who have limited metastasis in the celiac axis, superior pancreatic, or retroduodenal chains and may be cured by a D2 lymph node resection.75 Data from the Surveillance Epidemiology and End Results (SEER) database show that the number of nodes examined correlated with overall survival, potentially reflecting improved staging in these patients.76 Japanese researchers advocate complete lymph node removal to improve the rates of local control and survival. Several nonrandomized clinical trials suggested that extended lymphadenectomy may improve survival.77–78,79–80 Others81 reported that increasingly radical lymphadenectomies failed to improve survival or reduce the risk of locoregional failure. At least four prospective randomized trials of lymphadenectomies have been reported74,82–86,87 and show no survival advantage with more extensive lymph node dissection. Additionally, morbidity and mortality rates have been significantly higher for patients undergoing more extensive nodal dissection. An even more extensive lymph node dissection may entail para-aortic nodal dissection. However, a Japanese trial comparing D2 lymphadenectomy versus the same with para-aortic nodal dissection did not show any differences in overall or relapse-free survival between the groups, indicating the later approach should not be used in patients with curable gastric cancer.88 However, other important principles of lymph node dissection have been elucidated through these trials. The first is that as more lymph node areas are dissected and as pathologic lymph node evaluation is more rigorous, considerable stage migration occurs. This stage migration produces an apparent improvement in stage-specific survival without improvement in survival in the group overall.
TABLE 58.4 POSITIVE MARGINS IN RESECTED LONGITUDINAL GASTRIC SPECIMENS

TABLE 58.5 PATTERNS OF FAILURE AFTER “CURATIVE” RESECTION OF GASTRIC CANCER
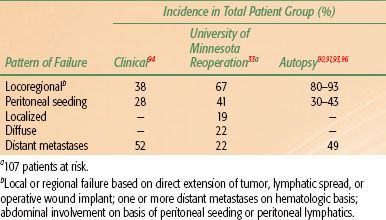
TABLE 58.6 PATTERNS OF LOCOREGIONAL FAILURE AFTER RESECTION OF GASTRIC CANCER
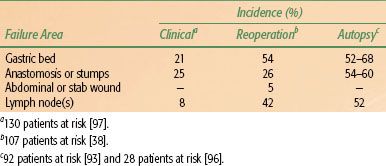
Failure Patterns After Surgical Resection
Local failures in the tumor bed and/or regional lymph nodes and distant failures by hematogenous or peritoneal routes are common mechanisms of failure after “curative” resection in clinical, reoperative, and autopsy series38,89, 90–92,93 (Tables 58.5 and 58.6). For GE junction lesions, the liver and lungs are common sites of distant metastases. With gastric lesions that do not extend to the esophagus, the initial site of distant metastasis is usually the liver, and many failures could be prevented if an effective abdominal treatment could be combined with treatment to the primary site. In the series of Landry et al.,94 50 of 88 (57%) failing patients had disease progression within the abdomen only. Abdominal treatment also could address peritoneal seeding, which occurs in 23% to 43% of postgastrectomy patients.89–92,93,94,95,96,97
Locoregional failures occur commonly in organs and structures of the gastric bed and in lymph nodes (Table 58.6). Clinically detectable locoregional recurrence following surgical resection alone generally exceeds 25%. However, reoperation and autopsy series have suggested that these rates are much higher.98 Failures in the anastomoses, gastric remnant, or duodenal stump also are frequent, as suggested by the incidence of positive longitudinal resection margins (Tables 58.4 and 58.6). As is true for most sites, clinical series underestimate the true incidence of locoregional failure when compared with reoperative or autopsy series (Table 58.5). Progressive extension of the operative procedure to include routine splenectomy, omentectomy, and radical lymph node dissection neither improved survival nor decreased the incidence of locoregional failures in the University of Minnesota series.38,96 Subsequent failure in areas of initial node dissection occurred frequently, even with radical node dissections38 (Fig. 58.3). The high rate of regional node relapse provides a partial explanation for the lack of survival benefit with a D2 (extended lymphadenectomy) versus D1 (limited lymphadenectomy) node dissection in the phase III surgical trials discussed previously.
Indications for Radiation Therapy
The results of the U.S. Gastrointestinal Intergroup Gastric Adjuvant Trial has changed the standard of care in the United States to the use of both chemotherapy and radiation therapy in the postoperative setting for patients with disease extension through the gastric wall and/or with nodes positive for tumor (discussed later).99 Postoperative irradiation plus concurrent and maintenance 5-FU–based chemotherapy is recommended for patients with stage IB-IV and M0 gastric cancer.99 Quality control of irradiation field design was conducted during the cycle of chemotherapy given before the start of concurrent chemoirradiation. The up-front quality control provided the mechanism to correct most of the major or minor deviations (35% incidence) in irradiation field design before the start of treatment and resulted in only a 6.5% final major deviation rate.
Radiation therapy, usually administered with concomitant 5-FU–based chemotherapy, is also indicated for locally confined gastric cancer that either is not technically resectable or occurs in medically inoperable patients. In this setting, therapy can be administered with curative or palliative intent, depending on the clinical situation. Those who undergo gastric resection with incomplete tumor resection or have truly positive margins of resection also are managed appropriately by combined-modality postoperative therapy.
 RADIATION THERAPY TECHNIQUES
RADIATION THERAPY TECHNIQUES
Simulation
When gastric cancer patients are simulated, the radiation oncologist should know the extent of disease based on imaging (barium swallow, CT, PET) as well as endoscopic procedures. CT simulation is appropriate for treatment planning. During simulation, the patient is positioned, straightened, and immobilized on the simulation table. An immobilization device is used to minimize variation in daily setup. Arms are generally placed overhead, and knees are supported underneath the legs. The administration of oral contrast to delineate the stomach is generally used and may help define the extent of mucosal irregularity. It may be advisable to have the patient come in with an empty stomach. The patient is placed on the CT simulator in the treatment position, and a scan of the entire area of interest with margin is obtained. At minimum, 3- to 5-mm slices should be used, allowing accurate tumor characterization as well as improved quality of digitally reconstructed radiographs. If patients lose >10% of their body weight during therapy, consideration should be given to repeat CT planning. Arterial phase IV contrast is generally used to delineate mediastinal and abdominal vascular nodal basins, including the celiac axis, and to allow the radiation oncologist to discern normal vasculature from other adjacent normal structures, potential adenopathy, and so forth. The tumor (if the patient is surgically naïve) and vital structures are then outlined on each slice on the treatment-planning system, enabling a three-dimensional (3D) treatment plan to be generated. The use of respiratory gating or breath-hold techniques may help to reduce target motion with respiration and, therefore, avoidance of normal tissue irradiation associated with larger margins used in free-breathing approaches. Four-dimensional (4D) CT scan may be appropriate to assess tumoral motion, facilitating appropriate margin placement on the target volumes.
Treatment Planning
Target Design
For a detailed description of target and field design in proximal gastric cancer involving the GE junction, the reader is referred to Chapter 53. In the design of radiation fields for neoadjuvantly treated or locally unresectable gastric cancer (as well as in the re-creation of tumoral volumes in adjuvantly treated patients), it is important to define varying target volumes, including gross disease as well as potential areas of subclinical involvement (i.e., the gross tumor volume [GTV] and clinical target volume [CTV], respectively). Defining GTV (including re-creation of volumes in the adjuvant setting) is based on multiple studies, including endoscopic descriptions (from both esophagogastroduodenoscopy and endoscopic ultrasound) as well as cross-sectional imaging. Gastric wall thickening correlating to the GTV can frequently be visualized on diagnostic and radiation planning CT. Similarly, EUS appears to be the most reliable test in detecting lymphadenopathy related to nodal spread. The endoscopist should be encouraged to accurately define not only the primary disease extent on EUS but also depth of penetration and potential involvement of adjacent structures, which can also be used to help guide GTV delineation. Similarly, EUS may allow detection of lymph nodes that may not be appreciated on CT or PET imaging, and the endoscopist should describe the size as well as location (e.g., relationship to tumor or adjacent structures). Additionally, radiographic areas of lymphadenopathy should similarly be included in the GTV. In GTV design, basing potential nodal involvement (and therefore target volumes) on nodal size is problematic given that metastatic nodes may frequently be below the resolution of conventional imaging. Therefore, it is not appropriate to rely exclusively on imaging modalities to define areas of subclinical spread for gastric cancer, realizing established pathologic patterns of spread data is important in determining radiation field design.
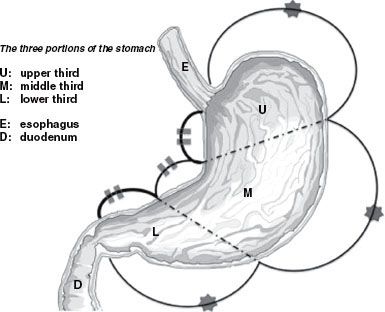
The identification of potential direct and nodal pathways for spread of subclinical disease (i.e., CTV definition) in gastric cancer is also of paramount importance. These areas vary significantly depending on site of origin of disease, making gastric cancer planning somewhat complex. As described previously, the stomach is characterized by a rich network of lymphatics that facilitates early lymph node spread of disease. Some authors have recommended dividing the stomach into three equal lengths (upper/proximal, middle, and distal), with tumors classified by location of the bulk of their masses in these respective sites. Therefore, practically speaking, the stomach can be divided into proximal (remembering that in the AJCC seventh edition staging system, involvement of the GE junction by proximal third tumors would be classified as an esophageal tumor), middle, or distal segments (Fig. 58.4).
Primarily based on extensive analysis of patterns of nodal spread from surgical series, Japanese investigators have developed lymph node station classifications (the Japanese classification of gastric carcinoma of the Japanese Gastric Cancer Association) that have been validated in other series (Fig. 58.2). Based on this system, general recommendations for CTV definition for tumors in varying parts of the stomach are as follows. Proximal third stomach tumors should include the contour of the stomach with exclusion of the pylorus and antrum (keeping a minimal margin of 5 cm from the GTV). Middle third tumors should include the contour of the stomach from the cardia to the pylorus. Distal third tumor CTVs should include the stomach except for the cardia/fundus, again keeping a minimal margin of 5 cm from the GTV. If pyloric/duodenal invasion is present, the CTV would be expanded along the duodenum with a margin of 3 cm from the tumor. Nodal volumes to be included are further described later, and it has been recommended that the CTV consist of a 5-mm margin around corresponding vessels. If target motion is not accounted for, the minimal recommended 3D margins to the CTV to obtain the internal target volume (ITV) are 1.5 cm in all directions, taking into account physiologic organ motion, particularly respiratory motion. The PTV can then be defined as the ITV volume plus a 3D margin of 5 mm.101
Field Design
Based on the likely sites of locoregional failure (Table 58.6), in resected patients, the gastric/tumor bed, anastomosis and gastric remnant, and regional lymphatics should be included in most patients.38,93,96,97,100 Major nodal chains at risk include the lesser and greater curvature, celiac axis, pancreaticoduodenal, splenic, suprapancreatic, porta hepatis, and, in some, para-aortics to the level of mid-L3.
FIGURE 58.4. Subdivision of the stomach into equal lengths along greater and lesser curves. (From Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol 2009;92:164–175, with permission from Elsevier.)