Anal Cancer
The standard treatment for primary squamous cell cancers of the anal canal is chemoradiation, using concurrent radiation therapy (RT), 5-fluorouracil (5-FU), and mitomycin-C (MMC), with surgery reserved for residual cancer. This combination, developed empirically as preoperative adjuvant therapy in the 1970s,1 has evolved to become the standard of care. Trials of other chemotherapy agents in combination with radiation have so far not improved results. Currently, about 65% of those with cancer confined to the pelvis will survive 5 years or more and about 75% will retain anorectal function.
In this chapter, the emphasis is on the management of squamous cell cancer of the anal canal. Note is also made of the role of RT in the treatment of squamous cell cancers of the perianal skin and of adenocarcinomas of the anal region.
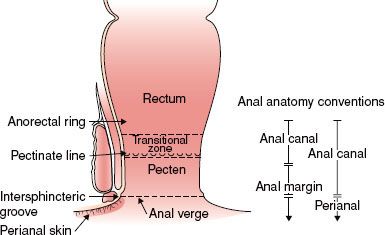
FIGURE 62.1. Anatomy of the anal region.
 ANATOMY
ANATOMY
The anal canal is 3 to 4 cm in length, the posterior wall being longer than the anterior. The canal ends superiorly at the palpable upper border of the anal sphincter and puborectalis muscle of the anorectal ring. The distal end at the anal verge is the level at which the walls of the anal canal come in contact in their normal resting state; it approximates the palpable groove between the lower edge of the internal sphincter and the subcutaneous part of the external sphincter and the junction with true skin. The American Joint Committee on Cancer Clinical Staging (AJCC)2 and the International Union Against Cancer (UICC)3 recommend this definition rather than a convention used by some centers under which carcinomas that are above or exactly astride the dentate line are classified as anal canal tumors and those lying mainly or entirely below that line are called anal margin tumors (Fig. 62.1). The terms anal margin and perianal skin are now generally used interchangeably. Perianal carcinomas are arbitrarily considered to be cancers arising from the skin within a 5-cm radius of the anal verge.
Four different types of epithelium are found within the anal region.4 The perianal skin is similar to hair-bearing skin elsewhere. At the anal verge, the skin blends with a pale-colored zone, sometimes called the pecten, lined by modified squamous epithelium that lacks hair or glandular structures. This squamous zone merges just below the dentate or pectinate line, which marks the mucosal folds of the anal valves, with a transitional epithelium that incorporates features of rectal, urothelial, and squamous epithelium. The purplish red–colored transitional zone extends proximally for about 2 cm until the pinker glandular mucosa of the rectum becomes dominant.
The major lymphatic pathways flow to three lymph node systems. The perianal skin, the anal verge, and the canal distal to the dentate line drain predominantly to the superficial inguinal nodes, with some communication to the femoral nodes and to the external iliac systems. Lymphatics from around and above the dentate line flow with those from the distal rectum to the internal pudendal, hypogastric, and obturator nodes of the internal iliac systems. The proximal canal drains to the perirectal and superior hemorrhoidal nodes of the inferior mesenteric system. There are numerous lymphatic connections between the various levels of the anal canal, and an intramural system links the lymphatics of the canal with those of the rectum.5
The veins of the anal canal connect with both the systemic and the portal venous systems. Venous plexuses, which lie in and surround the mucosal and muscular structures of the anal wall, anastomose around the junction of the anal verge and distal canal. The veins draining the inferior parts of these plexuses communicate with the systemic venous system via the internal pudendal and internal iliac veins, and those from the superior canal flow predominately to the inferior mesenteric vein and then to the portal system.5
Anorectal continence is mediated by both cerebrospinal nerves and the autonomic system. The smooth muscle of the internal sphincter is supplied by parasympathetic fibers from the second, third, and fourth sacral segments as well as sympathetic fibers from the hypogastric plexus. The upper canal has selective sensitivity for intraluminal differences in pressure, and the autonomic nerves mediate both the inhibitor and facilitator reflexes of the internal sphincter. The striated muscle of the external sphincter is under voluntary control and innervated by the internal rectal nerve, a branch of the pudendal nerve arising from the second, third, and fourth sacral nerves. The internal rectal nerve also transmits pain, touch, and other sensations from the anal lining below the dentate line and from the perianal skin.5
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
The most recent revision of the World Health Organization (WHO) classification of anal tumors, published in 2000, describes intraepithelial and invasive neoplasms.6 The term anal intraepithelial neoplasia (AIN) is applied to precancerous changes in the epithelium of the anal canal and perianal skin.7
For invasive cancers, the term squamous cell carcinoma is applied to all the various subtypes, squamous, large-cell keratinizing and nonkeratinizing, and basaloid, cloacogenic, and transitional, all of which were considered separate entities in the previous classification.6 Clinicians have for some years grouped these various subtypes as epidermoid cancers because of their similar natural history; the term epidermoid is not used in the WHO classification. About 85% to 90% of primary anal canal cancers are squamous cell type. The remaining 10% to 15% are predominantly adenocarcinomas, most of which arise from anal glands or within anal fistulae. Adenocarcinomas from the rectal-type mucosa in the upper canal are classified as primary rectal cancers. The WHO system retains as separate entities rare variants such as squamous cell carcinoma with mucous microcysts, small cell, and undifferentiated cancers.
Primary cancers of the perianal skin are similar to cancers of the skin in other sites. Most are squamous cell cancers, with occasional basal cell cancers and skin adnexal adenocarcinomas.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Anal cancers are about one-tenth as common as cancers of the rectum. Cancers arise in the canal with three to four times the frequency of perianal cancers. In North America and Europe, anal canal cancers are more common in women than in men, although this difference is decreasing8; perianal cancers occur with about equal frequency in both sexes. The annual age-adjusted rates per 100,000 for squamous cell cancers of the anus, anal canal, and anorectum in the U.S. Surveillance, Epidemiology, and End Results (SEER) registry for 1995 to 2004 were 0.89 for males and 1.14 for females.8 The incidence varies widely in different parts of the world, but has been increasing in North America and Europe over the past 40 years.8 The annual incidence of invasive and intraepithelial neoplasia almost doubled in men and rose by about half in women between the periods 1973 to 1979 and 1994 to 2000.9 The risk of anal cancer increases with age; the median age at diagnosis is from 60 to 65 years.
 RISK FACTORS
RISK FACTORS
The most significant risk factors so far identified are sexually transmissible viruses, immunosuppression, and tobacco smoking. The role of sexual practices and sexually transmissible agents has been investigated intensively. Several mucosotropic types of human papilloma virus (HPV) are linked to cancer and precancerous lesions in the anogenital epithelium.10–12 Subtypes of HPV with a high risk of association with cancer are type 16 in particular, and, to a lesser extent, types 18, 31, 33, 35, and others.10–12 These high-risk HPV types have been found in about 85% of anal squamous cancers in many series,10,11 more commonly in cancer of the canal than of the perianal skin.11,13 Some geographic variation was noted in the types of high-risk HPV identified.11,14 Case control studies suggest that a history of multiple sexual partners in homosexual or heterosexual relationships or of unprotected anal intercourse in males and in females are predictive of an increased risk of AIN and invasive anal cancer.15 Compared to the overall annual incidence of anal cancer in white males in the United States of approximately 0.7 per 100,000 in the early 1980s, the estimated incidence in the male homosexual population, prior to the human immunodeficiency virus (HIV) epidemic, ranged from about 12 to 37 per 100,000.16 This rate increased to about 70 per 100,000 among HIV-positive male homosexuals.17
Compromise of cell-mediated immunity reduces the body’s ability to prevent or eliminate infection by viruses such as HPV. An increased risk of anal cancer is associated with at least two situations in which cell-mediated immunity is significantly altered: infection with HIV and iatrogenic suppression of immunity in organ transplant patients. Interactions between HPV and HIV are complex. Although anal cancer has not been designated an acquired immunodeficiency syndrome (AIDS)-defining condition, data from the U.S. AIDS Cancer Registry linkage study showed that the rate of HPV-associated cancers and precursors was increased in HIV-infected persons for all anogenital sites compared with the general population. The relative risk for anal cancers was 6.8 in women and 37.9 in men.18 A national cohort study in Sweden of 5,931 patients who had undergone organ transplantation showed a 10-fold excess risk of developing anal cancer.19 Whatever the cause of suppression of cell-mediated immunity, those affected have increased rates of HPV infection, higher progression rates from normal epithelium to AIN and from lower to higher grade AIN, and lower rates of clearance of HPV and regression from abnormal to normal epithelium.20,21
The increasing effectiveness of antiretroviral therapy has led to longer survival of HIV-infected patients and an increased number with anal cancer.22,23 There was a 3-fold increase in incidence in the HIV-positive population from the period prior to the introduction of highly active antiretroviral therapy (HAART) in about 1996 and the later HAART era.23 Although there was a decrease in the incidence of Kaposi’s sarcoma and lymphoma by a few years after HAART became available, at the time of the review there had been no significant reduction in the incidence of less common malignancies such as anogenital cancers.24 Early success of prophylactic vaccines against HPV in both men and women suggests that the incidence of anal cancer can be reduced in the future.25,26
Tobacco smoking is associated with up to a 4-fold increase in risk in several case-control studies. Current smokers are at greater risk than past smokers.15,27 Benign conditions such as fistulae, fissures, and hemorrhoids do not appear to predispose to cancer.28 Chronic anal inflammation due to inflammatory bowel disease has also been discounted as a risk factor.29
 NATURAL HISTORY
NATURAL HISTORY
Most squamous cell cancers of the anal region, especially the canal, are believed to be preceded by high-grade AIN.7 However, it has been estimated that no more than 1% of cases with AIN develop invasive cancer per year,30 a rate lower than that described for cervical intraepithelial neoplasia. Anal dysplasia recurs frequently despite excision, laser ablation, or topical therapies, presumably because of incomplete treatment or persistence of the HPV infection with which dysplasia is associated.22 The natural history of AIN coexisting with anal cancers exposed to radiation, with or without chemotherapy, is unknown.
Squamous cancers of the anal canal spread most commonly by direct extension and lymphatic pathways. Hematogenous metastases are less common. Direct invasion from the anal mucosa into the sphincter muscles and perianal connective tissue spaces occurs early; in a series of 137 cases, cancers were confined to the mucosa and submucosa at diagnosis in only 12%, and to the sphincter muscles, without regional lymph node involvement, in only 34%.31 In about half the cases, anal cancers extend into the rectum or perianal skin. Invasion of the vaginal septum and vaginal mucosa is more common than invasion of the prostate gland, but anovaginal fistulas occur in fewer than 5% of women. Extensive tumors may infiltrate the pelvic walls.
Lymphatic invasion occurs relatively early. The overall risk of regional nodal involvement at diagnosis is about 25%.32,33 Pelvic lymph node metastases were found in as many as 30% of patients treated by abdominoperineal resection.31,34,35 In an illustrative series, metastases were present in the superior hemorrhoidal nodes in 25% (15 of 61); in the external iliac, obturator, or hypogastric nodes in 30% (8 of 27); and in the inguinal nodes in 16% (12 of 74) of patients.35 Inguinal metastases were detected clinically in up to approximately 20% of patients at initial diagnosis and were present subclinically in a further 10% to 20%.31,35,36,37 Nodal metastases were associated with 30% of cancers confined to the sphincter muscles, and 60% of those that had extended through the sphincters or were more poorly differentiated.34 Lymphatic metastases increase in frequency with progressive enlargement of the primary cancer.34,38
Extrapelvic metastases are identified at the time of first presentation in <10% of patients. They are found most frequently in the liver, lungs, and para-aortic nodes. Relapse after initial treatment is more common in the area of the primary tumor and the pelvic lymph nodes than in extrapelvic organs. Locoregional relapse rates of up to about 30% are common. Failure outside the pelvis occurs in up to about 20%.31,39,40,41
Perianal cancers tend to grow locally and may extend into the anal canal. When the site of origin is in doubt, it is conventional to classify the cancer as arising in the anal canal. The ipsilateral inguinal nodes are the most common site of metastasis and are involved in from 5% to 20% of cases. Extrapelvic metastases are uncommon except in locally advanced cancer or those with nodal metastases.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
The symptoms of anal cancer are nonspecific, contributing to delay in presentation by the patient and in diagnosis by the physician of more than 6 months in a third of patients.42,43 Bleeding and anal discomfort are the most common symptoms and are reported by about half the patients.44 Less common complaints include awareness of an anal mass, pruritus, and anal discharge. Pain is uncommon but may be severe. In patients with tumors in the proximal canal, there may be an alteration in bowel habits, but this is uncommon with distal cancers.44 Occasionally, asymptomatic tumors are found during physical examination or in the course of investigation of an enlarged inguinal node. Unsuspected microinvasive carcinoma is sometimes found in mucosa removed at hemorrhoidectomy.45
Small carcinomas are often nodular or plaque-like, but larger tumors are more typically ulcerated and infiltrative. Coexisting benign conditions such as anogenital warts, hemorrhoids, and anal fissures may be present. Anal sphincter tone is usually preserved and may be increased by painful spasm. Gross fecal incontinence resulting from sphincter destruction occurs in <5% of patients, although some fecal soiling is often reported. Similarly, vaginal fistulas are uncommon. Rarely, extrapelvic metastases may be the only symptomatic feature or finding.
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
The history and physical examination should stress features that delineate the extent of the primary tumor, including anal sphincter competence and possible invasion of adjacent organs. A biopsy of the primary tumor is necessary to establish the diagnosis and the histologic type. General anesthesia may be needed to permit detailed pelvic and anorectal examination, which should include proctoscopy. Sigmoidoscopy and colonoscopy, although often performed, infrequently disclose colonic pathology.46 Physical examination should include detailed examination of the genital region, especially in patients who give a history of previous anogenital area dysplasia or cancer, genital warts, anal-receptive intercourse, or are HIV positive.
Only the inguinal and low perirectal lymph nodes are accessible to clinical examination. Because lymph node enlargement may be caused by reactive hyperplasia in as many as half of those with palpable inguinal nodes, clinically suspicious nodes should be assessed histologically by needle biopsy or simple excision.44 Metastases in the internal iliac and superior hemorrhoidal node chains, about half of which are <0.5 cm in diameter,47 cannot be identified reliably by current techniques of lymphangiography, pelvic lymphoscintigraphy, computed tomography (CT), magnetic resonance imaging (MRI), or transanorectal ultrasonography. MRI is considered the most accurate method for assessing the primary cancer and pelvic nodes.48 Both fluorodeoxyglucose (FDG) positron emission tomography (PET) with CT and inguinal sentinel lymph node biopsy (ISLN) have been used to identify inguinal node metastases and to refine RT plans.49–51 However, a comparison of FDG-PET-CT and ISLN biopsy in 27 patients found several false-positive PET studies (4 of 7).52 Also, late inguinal node metastases have been reported after a previous negative ISLN biopsy.53 Both FDG-PET-CT and ISLN biopsy are as yet of unproven value in the initial management of anal cancer.
Thoracic, abdominal, and pelvic CT and pelvic MRI identify liver or lung metastases or enlarged nodes. If the diagnosis is uncertain, image-guided biopsy should be considered. Skeletal studies are not indicated in the absence of focal symptoms.
Examination of blood and serum should include full blood count, renal and liver function tests, and, if any risk factors are present, assessment of HIV antibody status.
 STAGING
STAGING
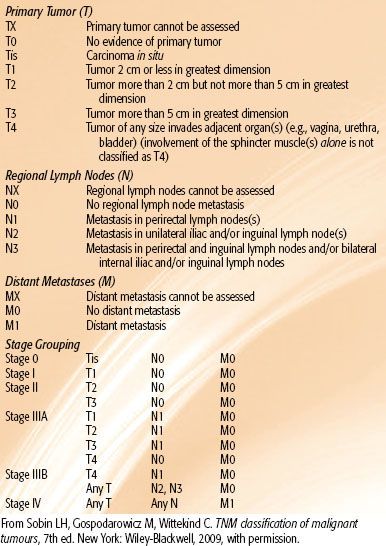
The staging systems for anal canal and perianal cancers most commonly used are those proposed by the UICC3 and the AJCC2 (Tables 62.1 and 62.2). Most authors continue to report results by T (tumor) category or N (node) category rather than by composite TNM (tumor, node, metastasis) stages. Under the UICC and AJCC systems, the regional lymph nodes for anal canal cancer are the perirectal, internal iliac, and inguinal nodes. Spread to all other pelvic node groups, including the external iliac, common iliac, and sigmoid nodes, is classified as metastasis (M1). For perianal cancers, the only regional nodes are the ipsilateral inguinal nodes.
In a review of 19,199 patients with squamous cell carcinoma of the anal canal, diagnosed between 1985 and 2000 and recorded in the U.S. National Cancer Data Base (NCDB), the stage distribution was stage I: 25.3%, II: 51.8%, III: 17.1%, IV: 5.7%. Tumors were >5 cm in size (T3) in at least 20.6% (tumor size in T4 category not available). Nodal involvement was present in 21.8% of those for whom information on nodal status was recorded.33
TABLE 62.1 ANAL CANAL TNM CLASSIFICATION
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
Tumor Factors
Features related to the anatomic extent of disease generally provide the most prognostic value.54 The most adverse factor for survival is the presence of extrapelvic metastasis.55,56–57 When anal cancer is confined to the pelvis, the size of the primary tumor is the most useful predictor for local control and preservation of anorectal function and survival.39,58,59 Involvement of regional lymph nodes is an adverse factor for survival but, in most series, not for control of the primary tumor.39,59
Among the 19,199 patients recorded in the NCDB between 1985 and 2000, the overall 5-year survival was 58%.33 Patients with distant metastases had a 5-year survival of 18.7% versus 59.4% for those without metastases. Patients with regional node metastases had a 5-year survival of 37.4% versus 62.9% in node-negative patients. Five-year survival rates by T category were T1: 68.5%, T2: 58.9%, T3: 43.1%, and T4: 34.3%. The survival rates by AJCC stage are shown in Figure 62.2.
In detailed analyses of data collected prospectively for Radiation Therapy Oncology Group trial RTOG-9811,59,60 tumor diameter >5 cm correlated with an increased likelihood of colostomy (hazard ratio [HR] 1.8; P = .008)59 (Table 62.3). A tumor size >5 cm and clinically positive nodes were each associated with significantly poorer 5-year disease-free and overall survival.60 These findings were combined into prognostic groups.60 These groups are shown in Figure 62.3, with additional data drawn from the analyses by Ajani et al.59,60 These groupings have not been verified prospectively.
TABLE 62.2 PERIANAL SKIN TNM CLASSIFICATION
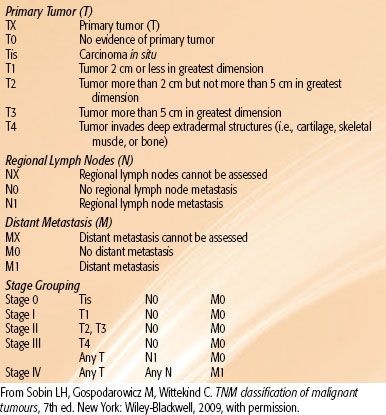
TABLE 62.3 PROGNOSTIC GROUPS: BASED ON DATA FROM RTOG TRIAL 9811
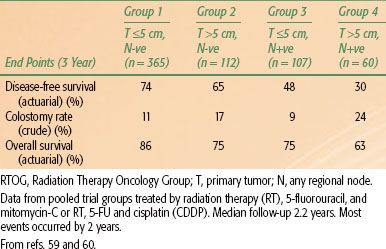
FIGURE 62.2. Five-year survival rates by American Joint Committee on Cancer Clinical Staging and the International Union Against Cancer stages, for 19,199 patients recorded in the U.S. National Cancer Data Base between 1985 and 2000. (From ref. 33, with permission.)
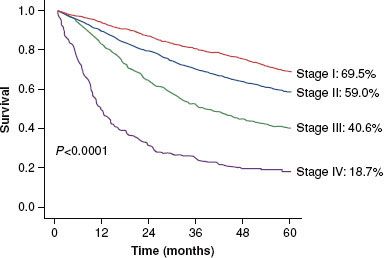
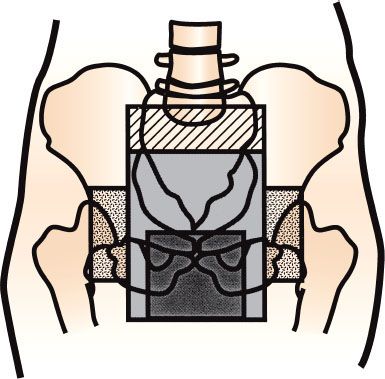
FIGURE 62.3. Example of radiation fields used to cover pelvic nodes and primary cancer. Upper border generally lowered from lumbosacral junction to lower border of sacroiliac joints part way through course. Fields are later reduced further to give higher dose to primary tumor. Separate anterior fields are applied to cover lateral inguinal nodes.
Patient Factors
Patient-related factors are not consistent between series. Age, performance status, gender, baseline hemoglobin level, and race have all been considered prognostic in one or more series. Patients who continue to smoke tobacco may be at greater risk of local relapse.61 In some series of HIV-positive patients, high viral load, low lymphocyte CD4-positive counts, and AIDS have been prognostic of poor local tumor control and survival, and, in some series, of impaired tolerance of RT and chemotherapy.62–63,64
Biochemical and Molecular Factors
A survey of nearly 50 reports on cytogenetic, flow cytometric, immunohistochemical, and other factors considered that these studies offered some insights on pathogenesis but did not help with selection of treatment or provide guidance on prognosis.65 Recent studies suggested that biomarkers such as p53,66 Ki-67, nuclear factor kappa B, 5HH, and Gli-1,67 may be associated with locoregional control or disease-free survival. High levels of MCM7 protein detected in gene expression analysis were associated with improved cancer survival in one series.68 None of these suggested markers has yet been validated in prospective studies.
Treatment Factors
Early response, and the extent of response, to chemoradiation were correlated with survival in several multivariate analyses.54,69,70 An association with total radiation dose or with treatment duration, while expected, has not been demonstrated consistently for either locoregional control or survival. A daily fraction size of 2.5 Gy appeared to be associated with increased acute and late toxicity, compared with 2-Gy fractions.39
TABLE 62.4 SELECTED RESULTS OF PHASE III TRIALS
 TREATMENT OF ANAL CANCER
TREATMENT OF ANAL CANCER
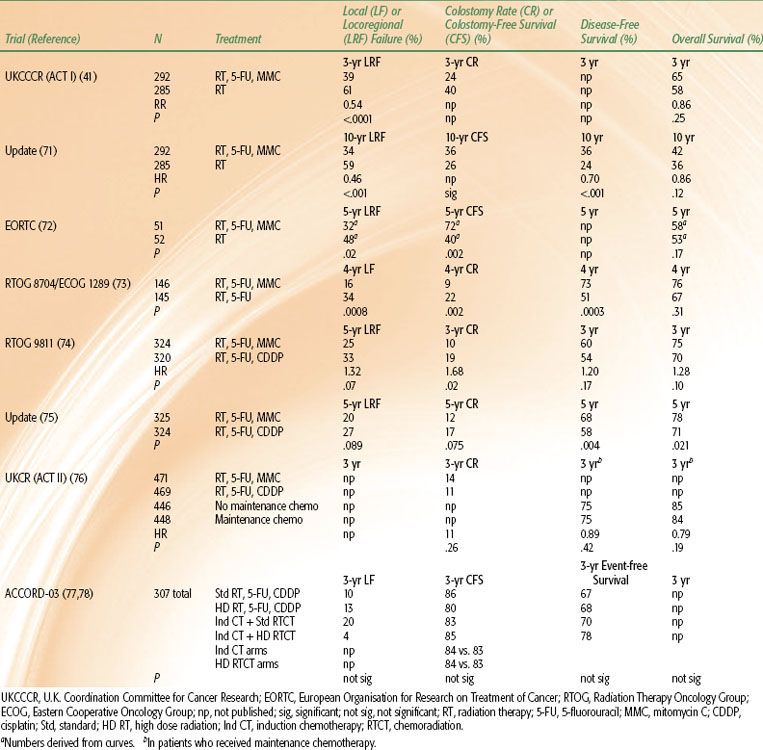
Although squamous cell cancer of the anal canal is a relatively uncommon malignancy, several multicenter randomized trials have been completed successfully. These have established RT, 5-FU, and MMC, with surgery reserved for failure, as the standard against which other treatments should be compared. These trials are outlined here and in Table 62.4.41,71–78 The original references should be consulted for full details of patient eligibility, treatment regimens, outcomes, and statistical analyses. There have also been more than 100 nonrandomized studies, many of which provided hypotheses subsequently tested in the randomized studies, or information that guided the development of technical aspects of radiation treatment.79
It is of note that the combination of RT and chemotherapy has supplanted surgery as the preferred treatment for anal canal squamous cell cancer without formal comparison in a randomized trial. Nonrandomized comparisons of radical resection with this combination of RT, 5-FU, and MMC32 or with radiation therapy alone80 have shown the ability of radiation-based regimens to produce survival rates at least equal to those of surgical series, while allowing preservation of anorectal function in the majority of patients.
 COMBINED RADIATION AND CHEMOTHERAPY
COMBINED RADIATION AND CHEMOTHERAPY
Radiation, 5-Fluorouracil, and Mitomycin-C Versus Radiation Alone
In both the United Kingdom and Europe in the 1970s, more so than in North America, there was already some acceptance of RT as the primary treatment for anal cancer81; this facilitated the development of the first multi-institutional randomized trials in which RT alone was compared with RT plus chemotherapy. The randomized trials conducted by the U.K. Coordination Committee for Cancer Research (UKCCCR)41 and the European Organisation for Research on Treatment of Cancer (EORTC)72 both demonstrated significant improvement in control of the primary cancer and regional nodes and in colostomy rate or colostomy-free survival in patients who received RT, 5-FU, and MMC. Overall survival rates were not improved significantly in the initial reports. However, in a later report of the UKCCCR trial after a median of 13 years’ follow-up, locoregional control, colostomy-free survival, disease-free survival, and deaths due to anal cancer all significantly favored chemoradiation.71 The absolute difference in risk of dying from all causes was 5.1% lower in the chemoradiation group at 5 years and 5.6% lower at 12 years from randomization. This difference approached statistical significance (HR 0.86; 95% confidence interval [CI], 0.70 to 1.04; P = .12).71
The UKCCCR trial included 577 patients with all stages (UICC staging system, 1987 edition) of squamous cell cancer of the anal canal (75% of patients) or anal margin (23%).41 The radiation dose was 45 Gy in 20 to 25 fractions in 4 to 5 weeks. Those randomized to chemotherapy received 5-FU (1,000 mg/m2/day for 4 days or 750 mg/m2/day for 5 days) by continuous peripheral intravenous infusion in the first and final weeks of radiation treatment, plus MMC (12 mg/m2) by bolus intravenous injection on day 1 of the first course of chemotherapy. If the primary tumor had not regressed by at least 50% by 6 weeks after treatment (as occurred in 10% in each group), surgery was recommended; otherwise, the patients received an additional 15 Gy in 6 fractions by a perineal field or 25 Gy over 2 to 3 days by iridium-192 implant. The locoregional recurrence rates at 3 years were 61% after RT and 39% after RT, 5-FU, and MMC (P <.0001). The overall survival rates at 3 years were 58% and 65% (P = .25), respectively. There were six (2%) deaths due to treatment in the combined-modality arm and two (0.7%) in the irradiation-alone arm. Acute toxicity, other than hematologic, was considered comparable in each group. In the first few years after treatment, surgery that included colostomy was necessary for management of toxicity in 10 patients (3.5%) in each group.41 Late toxicity events (severity was not graded) after a median of 13 years’ follow-up were similar in each treatment group.71
In the EORTC study, 103 patients with advanced cancers of the anal canal were randomized in a trial of similar design.72 The radiation dose was 45 Gy in 25 fractions over 5 weeks. Chemotherapy included 5-FU (750 mg/m2/day for 5 days) in weeks 1 and 5 of radiation, and a single dose of MMC (15 mg/m2) by bolus intravenous injection on day 1 of the first course of 5-FU only. After 6 weeks, boost irradiation of 15 Gy (if complete clinical response to previous treatment had occurred) or 20 Gy (after partial response) was given by external-beam or interstitial irradiation. The 5-year locoregional recurrence rate was 48% after RT and 32% after RT, 5-FU, and MMC (P = .02). The difference between overall survival rates (53% and 58%, respectively) was not significant. One of 51 patients who received combined modality treatment died of toxicity. Otherwise, acute and late toxicity rates did not differ markedly. These two trials established RT, 5-FU, and MMC as the standard first-line treatment.
Radiation, 5-Fluorouracil, and Mitomycin-C- Versus Radiation and 5-Fluorouracil
The RTOG and Eastern Cooperative Oncology Group (ECOG) established in a randomized trial that the combination of MMC with 5-FU and RT is more effective than 5-FU alone with RT.73 This study was undertaken to determine whether MMC was a necessary component of the protocol and whether the hematologic toxicity of that drug could be avoided. Two hundred and ninety-one patients with cancers of the anal canal of any T and N category (RTOG staging system) who did not have evidence of extrapelvic metastases received 45 Gy to 50.4 Gy in 25 to 28 fractions over 5 weeks, plus 2 courses of 5-FU (1,000 mg/m2/day by continuous peripheral intravenous infusion) over 4 days, with or without MMC (10 mg/m2 by bolus intravenous injection) on the first day of each course of chemotherapy. Chemotherapy was administered in weeks 1 and 5 of radiation therapy. All patients underwent biopsy of the primary tumor site 6 weeks after treatment. Biopsies were positive in 15% of those who received 5-FU only and in 8% of those who received both MMC and 5-FU (P =.14). Patients with positive biopsies had the option of receiving an additional 9 Gy in 5 treatments concurrently with a 4-day infusion of 5-FU (1,000 mg/m2/day) and a single injection of cisplatin (CDDP) (100 mg/m2) if it was thought that anal function might still be salvaged. At 4 years, the rates of local failure (16% vs. 34%; P = .0008) and of colostomy (9% vs. 22%; P = .002), significantly favored treatment with radiation, 5-FU, and MMC. There was no significant difference in overall survival rates (76% with RT, 5-FU, and MMC vs. 67%); however, the disease-free survival rate (73% vs. 51%; P = .0003) was improved by the three-agent combination. Acute hematologic toxicity was more common in the patients who received MMC, but the rates of other acute and late toxic effects were similar in each treatment group. Four of 146 (2.7%) patients who received both 5-FU and MMC suffered fatal toxicity as did 1 of 145 treated with RT and 5-FU alone. On the evidence from this trial, MMC was retained in combination with RT and 5-FU.
Radiation, 5-Fluorouracil and Mitomycin-C Versus Radiation, 5-Fluorouracil, and Cisplatin
Several pilot studies suggested high tumor response rates to concurrent, induction, or induction plus concurrent 5-FU and CDDP with RT.82–88 Cisplatin was known to be effective against squamous cell cancers in several other sites and to be associated with stronger laboratory evidence as a radiosensitizer than MMC.
The RTOG elected to evaluate a strategy of CDDP-based induction chemotherapy intended to downsize the primary tumor and nodal metastases prior to concurrent chemoradiation. This trial included a higher total radiation dose than the earlier RTOG randomized trial,73 based on analysis of nonrandomized studies that suggested a radiation dose–control relationship,89,90 and on pilot studies which had demonstrated that a proportion of patients could tolerate uninterrupted radiation schedules of up to 59.4 Gy over 6.5 weeks with either concurrent MMC with 5-FU91,92 or CDDP with 5-FU.83
In RTOG-9811,74 one patient group received 59 Gy in 6.5 weeks (45 Gy in 1.8-Gy fractions, followed without interruption by 14 Gy in 2-Gy fractions), together with concurrent 5-FU (1,000 mg/m2/day) by continuous infusion on days 1 to 4 and 29 to 32 plus MMC 10 mg/m2 intravenous bolus on days 1 and 29; the other group received 5-FU (1,000 mg/m2/day) days 1 to 4, 29 to 32, 57 to 60, and 85 to 88 plus CDDP (75 mg/m2 bolus injection on days 1, 29, 57, and 85) with the same 59 Gy radiation schedule (start day 57). Acute grade 3 or 4 nonhematologic toxicity rates were 75% in each arm, but hematologic toxicity was higher in the MMC group (67% vs. 47%). No treatment-related deaths were reported. The rate of severe long-term toxicity was similar in each group (11% MMC vs. 10% CDDP). The first report of this trial (after median patient follow-up of 2.5 years) described a significantly higher rate of colostomy at 3 years in those who received CDDP rather than MMC plus 5-FU and RT. There were no significant differences in disease-free or overall survival rates.
After longer follow-up, the 5-year colostomy rates and locoregional failure rates showed trends in favor of 5-FU, MMC- and RT but were not significant. However, the 5-year disease-free (68% vs. 58%; P = .004) and overall survival rates (78% vs. 71%; P = .021) significantly favored RT, 5-FU, and MMC.75 The investigators concluded that RT, 5-FU, and MMC should remain the standard approach.
The second U.K. Anal Cancer Trial (ACT II) also has not identified a role for CDDP in addition to or in place of MMC. This trial has so far been reported in abstract only.76 The trial incorporates a double randomization, either to 5-FU and MMC or to 5-FU and CDDP concurrently with RT; and to 2 courses of adjuvant (or maintenance) 5-FU and CDDP or to no additional chemotherapy following concurrent chemoradiation. The primary end points were improvements in complete tumor response rates and disease-free survival rates. Complete tumor response rates at 6 months were similar: 94% (MMC arm) and 95% (CDDP arm). Three-year disease-free and overall survival rates were not improved in those who received adjuvant 5-FU and CDDP. MMC-treated patients had more severe hematologic toxicity, but rates of neutropenic sepsis were similar (about 3%).
A French intergroup conducted a factorial designed four-arm trial (ACCORD 03) that evaluated the addition of induction 5-FU and CDDP and of a higher dose of boost RT (20 Gy to 25 Gy vs. standard 15 Gy). The baseline standard RT schedule was 45 Gy in 25 fractions in 5 weeks. The outcome has been reported in abstract only.77,78 The actuarial 3-year colostomy-free survival rate (the primary study end point) ranged from 80% to 85% in the four arms, with no significant advantages for either experimental treatment. Local control, event-free survival, and overall survival rates were similar in each arm.
Other Drug–Radiation Combinations
In an effort to develop a simplified schedule, the U.K. National Cancer Research Institute Anal Subgroup conducted a phase II trial of RT (50.4 Gy in 28 fractions in 5.5 weeks), a single intravenous injection of MMC (12 mg/m2 on day 1) and oral capecitabine (825 mg/m2 twice daily on each RT treatment day).93 The schedule was described as well tolerated with acceptable compliance (although only 58% received the full planned doses). The overall tumor response rate after treatment was 90% (complete in 24 of 31 and partial in 4 of 31). Although this use of oral capecitabine is promising and removes the need for continuous intravenous infusions of 5-FU, at this time, delivery of both 5-FU and MMC intravenously remains standard.
The EORTC conducted a randomized phase II trial in which RT (36 Gy + 2-week gap + 23.4 Gy) was combined with either MMC and 5-FU or MMC and CDDP. The overall response rates at 8 weeks were 92% (34 of 37) to RT, MMC, and CDDP versus 80% (31 of 39) to RT, 5-FU, and MMC, although greater compliance to the full regimen was seen with RT, 5-FU, and MMC.94 It is not known whether this group has proceeded with a planned phase III trial.95
Because epidermal growth factor receptor (EGFR) is known to be overexpressed in many epithelial cancers, including squamous cell cancer of the anal canal,96 EGFR blockers are being evaluated. The ECOG is conducting a phase II study of RT, 5-FU, CDDP, and cetuximab in HIV-negative patients.95 The AIDS Malignancy Clinical Trials Consortium is performing a similar study in HIV-positive patients.95 Other anti-EGFR agents under study include Nimotuzumab and panitumumab.95 Initial laboratory studies suggest that EGFR and K-RAS mutation analysis are not likely to be useful as a screening test for sensitivity to anti-EGFR therapy in anal canal cancer.96
In Sweden, bleomycin was given concurrently with RT in nonrandomized studies, but no benefit was apparent.97–98,99 A pilot study in the United Kingdom in which all three of the major drugs investigated, 5FU, MMC, and CDDP, were combined with RT was not pursued further because of excessive toxicity.100
Conclusion
Much remains to be learned about the mechanisms of interaction between radiation and chemotherapy in the treatment of cancer. The synergistic interactions of various combinations of RT, 5-FU, MMC- and CDDP observed in some laboratory studies are difficult to evaluate clinically, and no trials designed to study such interactions have been performed. Also, there have not been formal comparisons of more prolonged, but less daily dose-intense, infusions of 5-FU with the 96-hour to 120-hour infusions generally favored, nor of bolus injections with continuous infusions of 5-FU or CDDP. In most series, the timing of delivery of chemotherapy each day relative to irradiation was not tightly controlled, and the importance of scheduling is not known.
The current standard chemoradiation combination is RT with two concurrent 96-hour or 120-hour continuous intravenous infusions of 5-FU and one or two bolus injections of MMC. The most effective doses have not been established. When patients are to be managed outside a clinical trial, it is suggested that a schedule used in one of the prospective randomized trials, for which there are data on efficacy and toxicity, be adopted.
 RADIATION THERAPY
RADIATION THERAPY
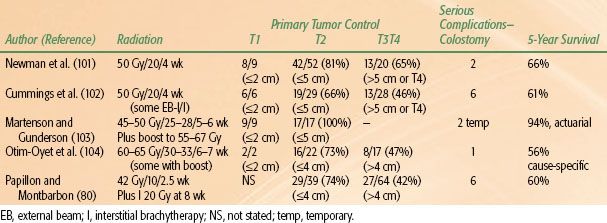
The use of RT alone, either brachytherapy or external beam, has been greatly reduced since the confirmation of improved outcome of combined modality therapy. Radiation alone is now recommended mainly to patients who are unable to undergo RT plus chemotherapy, or for the treatment of smaller cancers up to about 3 to 4 cm in size where the physician does not wish to add chemotherapy. As with combined RT and chemotherapy, primary tumor control is better with small tumors. Selected results, mainly from the period prior to the adoption of chemoradiation, are shown in Table 62.5.
 SURGERY
SURGERY
For Residual and Recurrent Cancer
Random biopsies from the site of the primary tumor, or abnormal nodes, shortly after chemoradiation have been advocated by some but are not necessary. Elective biopsies at predetermined times do not appear to lead to better results than can be achieved by biopsies directed only to areas suspected clinically of harboring residual or recurrent cancer. A negative biopsy does not exclude the possibility of cancer regrowth.105,106 Residual masses at the site of the original anal cancer may take several months to resolve fully after chemoradiation or RT alone.39,106 Most authors now recommend biopsy only when persistent cancer is suspected clinically.
Suspected residual or recurrent cancer at the primary site or in regional nodes should be confirmed histologically if possible. Full restaging imaging is recommended. FDG-PET scans may disclose otherwise unidentified cancer.107 Abdominoperineal resection or multivisceral pelvic resection is usually required. Survival rates are poor when R0 resection cannot be achieved.108,109 Locoregional recurrence is common, despite apparent R0 resection.108,110 Five-year survival rates following salvage surgery range from 30% to 50%, reflecting the varying criteria applied when selecting patients for surgery.108,109–110,111
Salvage for inguinal node metastases is usually by radical or selective inguinofemoral lymphadenectomy. Pelvic sidewall node metastases may not be resectable due to invasion of muscle or bone or neurovascular structures.
TABLE 62.5 SELECTED RESULTS OF RADIATION THERAPY ALONE