Stereotactic Radiosurgery and Radiotherapy
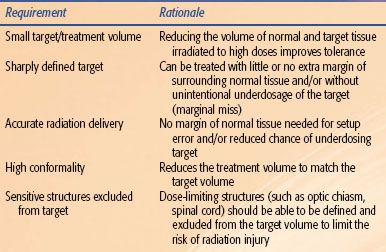
Stereotactic radiosurgery and radiotherapy are techniques to administer precisely directed, high-dose irradiation that tightly conforms to an intracranial target to create a desired radiobiologic response while minimizing radiation dose to surrounding normal tissue. These techniques exploit the fact that the radiation tolerance of normal tissue is volume dependent. With these techniques the complication risks for any radiation dose delivered are reduced by minimizing or eliminating the margin of normal tissue otherwise included in the radiation treatment volume with conventional radiotherapy techniques. In the case of radiosurgery, all of the irradiation is done in a single session or fraction, while in stereotactic radiotherapy, more than one fraction of irradiation is administered. Table 16.1 lists the key requirements for successful stereotactic irradiation. Advances in imaging, computers, and treatment planning in the last two decades have led to the development of a variety of different stereotactic radiosurgery/radiotherapy techniques and their wider applications. Successful clinical experience with intracranial radiosurgery for a variety of applications has led to a re-examination of radiobiology and exploration of both fractionated approaches and extracranial applications. Margin reduction with radiosurgery and fractionated stereotactic irradiation techniques makes target definition accuracy more critical. Drawing contours from different imaging techniques or by different physicians are approaches to reduce target definition error (Fig. 16.1).
FIGURE 16.1. Comparisons of acoustic schwannoma contours drawn from T1-contrast magnetic resonance (MR) images (eight axial images on the left side, with coronal and sagittal images on the right side) with contours drawn from T2 MR images (the darker eight axial images in the center). T1 contours can sometimes slightly overestimate extension into the internal auditory canal and unnecessarily include adjacent blood vessels.
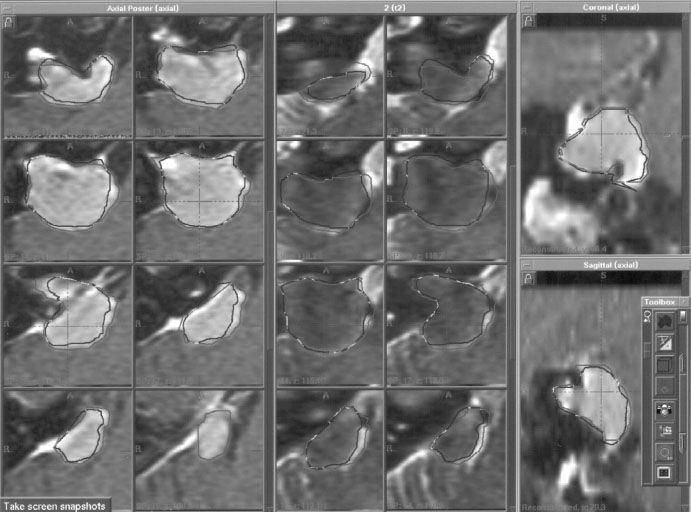
TABLE 16.1 KEY REQUIREMENTS FOR OPTIMAL STEREOTACTIC IRRADIATION
 TERMINOLOGY
TERMINOLOGY
Stereotactic refers to using a precise three-dimensional mapping technique to guide a procedure. The terminology used in stereotactic irradiation can be confusing. The term radiosurgery or stereotactic radiosurgery (SRS) is used for stereotactically guided conformal irradiation of a defined target volume in a single session. Radiosurgery or SRS can be delivered with Gamma Knife (Elekta Inc., Norcross, GA) modified LINAC radiosurgery systems (including CyberKnife [Accuray Inc., Sunnyvale, CA] and image-guided radiotherapy systems), tomotherapy, or proton beam systems. The term stereotactic radiation therapy (SRT) refers to stereotactically guided delivery of highly conformal radiation to a defined target volume in multiple fractions, typically using noninvasive positioning techniques. The term fractionated stereotactic radiosurgery (FSR) is limited to stereotactically guided high-dose conformal radiation administered to a precisely defined target volume in two to five sessions. Although it would have been less confusing to refer to this as hypofractionated SRT and reserve the term radiosurgery for single-fraction irradiation, the terminology is already in use. Adding intensity-modulated radiation therapy (IMRT) to the nomenclature can further complicate or confuse the terminology. Any radiation treatment plan that uses individual treatment beams that irradiate only part of the target at a time is IMRT. Strictly speaking, multiple isocenter radiosurgery (of a single target volume) meets the criteria for IMRT or stereotactic IMRT (SIMRT), but the term SIMRT is usually only used when multileaf collimators are employed. The terminology is useful to distinguish when the same linear accelerator is equipped either to treat with fixed circular collimators for radiosurgery (SRS or SRT mode) or to deliver IMRT using multileaf collimators (SIMRT mode).
 RADIOBIOLOGIC CONSIDERATIONS
RADIOBIOLOGIC CONSIDERATIONS
Prior to the introduction of radiosurgery, essentially all clinical irradiation was administered with radiation dose fractions between 1.2 and 3 Gy for intracranial targets. Extracranial targets were usually treated with 1.2- to 4-Gy fractions, with 6- to 8-Gy fractions used occasionally for treatment of bone metastases or malignant melanoma. Before the rapid adaption of radiosurgery into the clinic in the late 1980s, most radiation oncologists and radiation biologists believed that fractionating radiation treatment lessens the relative risk of injury to normal tissue compared with tumor in essentially all circumstances. Radiobiologic analysis of a limited number of malignant tumors in cell culture and clinical experience with conventionally fractionated radiotherapy of fast-growing malignant tumors established this radiobiologic dogma. Increasing the fractionation of radiotherapy for slow-growing benign tumors may not necessarily improve the balance between tumor control and radiation complication. Slow-growing benign tumors are difficult to study in either cell culture or animal models, so the effect of fractionation has not been well delineated. Stereotactic radiosurgery allowed clinicians to administer high single doses of radiation to intracranial targets with relative safety, thereby leading to a new appreciation of the underlying radiobiology. Laboratory studies suggest that the radiation response for the high-dose single fractions used in radiosurgery is predominantly related to the supporting endothelial cells.1 Pathology studies of benign and malignant tumors treated by radiosurgery also support a vascular response.2
Analysis of clinical data from radiosurgery to delineate dose–response relationships and define radiobiologic parameters is fraught with difficulties. Typical radiosurgery treatment plans use inhomogeneous dose distributions with the prescription isodose covering anywhere from 90% to 100% of the target volume. The absolute minimum dose to the target typically is 5% to 30% lower than the prescription dose. Contours of the same tumor/target volume or critical structures may vary slightly from one clinician to another. Using the linear-quadratic formula to extrapolate from experience with conventional radiotherapy with low-dose fractions to high-dose single fractions for radiosurgery appears problematic. Using single-fraction radiosurgery dose–response curves for arteriovenous malformation (AVM) obliteration and radiation injury to brain parenchyma and cranial nerves to calculate α/β ratios yields values of −30 to −60 rather than 2 to 3 as expected from conventional fractionated radiotherapy data.3,4 Comparing dose responses for fractionated SRT to radiosurgery is hampered by limited data with dose–response curves that have insufficient slopes for accurate comparison.
 RADIOSURGICAL TECHNIQUES
RADIOSURGICAL TECHNIQUES
Radiosurgery was originally envisioned to treat intracranial lesions by delivering a high dose of ionizing radiation in a single treatment session using multiple beams precisely collimated to the target inside the cranium. Advances in both imaging and computer technologies resulted in wider applications of radiosurgery. There are now a variety of different radiosurgery and SRT techniques available for intracranial and extracranial use.
Gamma Knife Radiosurgery
After prior experience with stereotactic treatment using orthovoltage radiotherapy and proton beam irradiation, Leksell and Larson created the first prototype of the gamma knife in 1967. The gamma knife uses a relatively hemispherical array of multiple fixed cobalt-60 beams (201 in most models) that are sharply collimated to create small, relatively spherical treatment volumes of varied diameter with sharp dose falloff. The earlier model originally was referred to as the U-style and contained cobalt sources arranged in a hemispherical array, including sources at the pole of the hemisphere. These units present challenging loading and reloading issues with the cobalt-60 sources, particularly with radiation protection. To eliminate this problem, the B-unit (Elekta, Inc., Norcross, GA) (after Bergen, Norway, the first site) was redesigned so that sources were arranged in an annular section of a hemisphere, similar to the Northern Hemisphere with the Arctic Circle excluded. In 1999, the model C version of the gamma knife was introduced with the option to use robotic positioning to set treatment coordinates. This expedites execution of multiple-isocenter treatment plans. Manual positioning is still needed for some targets far from the center of the head. The model 4-C, introduced in 2005, was equipped with enhancements designed to improve workflow, increase accuracy, and provide integrated imaging capabilities. The Perfexion model introduced in 2006 uses a larger patient aperture and internally mounted secondary collimators. Because of the larger patient aperture, it is able to treat all intracranial and even cervical spine targets quickly and efficiently with robotic positioning.
Rotating Gamma System
A radiosurgery device called the rotating gamma system (RGS) was developed in China. The rotating gamma system (OUR International Inc., Shenzen, China) employs 30 cobalt-60 radiation sources in a revolving hemispherical shell. The secondary collimator is a coaxial hemispheric shell with six groups of five different collimators to produce spherical treatment volumes of different diameter. The experience with this system is somewhat limited.
Proton Radiosurgery
The chief advantage of charged proton radiosurgery is that the beams stop at a depth related to the beam’s energy. Electron beams also use charged particles but lack the sharp beam edge of the proton beam. The lack of an exit dose and the sharp beam profile of protons allow target irradiation with lower integral doses than are delivered with photon (LINAC x-ray or cobalt-60 gamma) irradiation. An unmodified proton beam irradiation deposits increased energy in the last couple of millimeters of the path length. This area of increased ionization, where cell killing is even higher because of an increased radiobiologic effect, is termed the Bragg peak or Bragg-Gray peak. To allow homogeneous irradiation of targets greater than a millimeter or two, the Bragg peak is normally modulated or spread out throughout the target, essentially eliminating its effect. The first treatment of a malignant tumor by irradiation with a proton beam Bragg peak was carried out in 1957 and followed by functional neurosurgery for advanced Parkinson’s disease in 1958. Presently, proton beam irradiation is available at a limited number of centers because of the high cost of equipment and maintenance. If technical improvements and increased competition lead to continued cost reductions, proton beam irradiation will become increasingly used because of its dosimetric advantages.
LINAC Radiosurgery
The pioneering work of many researchers in the 1980s led to the gradual modifications of linear accelerators (LINACs) designed for conventional radiotherapy to be used for radiosurgery. LINAC technologies were modified by incorporating improved guiding (stereotactic) devices and methods to measure and improve accuracy of various components. Unmodified LINACs for conventional radiotherapy tend to deviate from alignment with the isocenter at different gantry angles. Most early LINAC-based radiosurgery techniques used multiple radiation arcs with circular secondary collimators to create spherical dose distributions for stereotactically defined three-dimensional targets. Improved hardware and advanced dose-planning software have been developed to enhance conformity. These include beam shaping with micromultileaf collimators and intensity modulation with inverse treatment-planning algorithms. Many LINAC-based systems such as XKnife (Radionics Inc., Burlington, MA), Novalis (BrainLAB, Heimstetten, Germany), the Peacock System (NOMOS Corp., Sewickley, PA), and CyberKnife (Accuray Inc., Sunnyvale, CA) are commercially available. The Peacock system uses inverse planning and multileaf wedge-generated intensity-modulated beams to obtain target conformity. The CyberKnife combines a miniaturized LINAC mounted on an industrial robot with a system for target tracking and beam realignment. This system uses a 6-MeV LINAC with different-sized circular collimators attached to a six-axis robotic manipulator. Stereotactic frames are not normally used for targeting. CyberKnife plans use multiple fixed-beam positions and multiple isocenters. Before the radiation is delivered from any beam position, the target position is tracked using an integrated x-ray image processing system, consisting of two orthogonal diagnostic x-ray cameras and an optical tracking system. During treatment, the image processing system acquires x-ray images of the patient’s body multiple times throughout the treatment, while stealth tracking software compares the actual images with the target images to correct alignment of the beam.
Tomotherapy
Tomotherapy, literally “slice” therapy, is a new form of radiotherapy that modifies the design of a diagnostic computerized tomography (CT) scan into a treatment delivery machine, thereby combining the precision of CT imaging with the radiation treatment. This is done by adding a LINAC megavoltage treatment beam to the rotating x-ray source and moving table design of the diagnostic CT unit, which normally uses only a kilovoltage diagnostic x-ray beam. Unlike traditional radiation therapy systems with a slow-moving external gantry designed for positioning individual beams onto the tumor from a few different directions, tomotherapy rapidly rotates the beam around the patient (and inside the housing of the unit), thus allowing the beam to enter the patient from many different angles in succession. Beam intensity modulation (IMRT) is possible through the use of a multileaf collimator system. The inclusion of CT imaging technology within the tomotherapy unit allows precise localization of the target before and during treatment.
LINAC Image-Guided Radiotherapy
The combination of diagnostic three-dimensional imaging with highly conformal treatment delivery in a single unit to maintain accuracy is the basis of various treatment techniques and processes collectively known as image-guided radiation therapy (IGRT). Although tomotherapy (which is also a type of IGRT) accomplishes this by modifying a CT unit into a megavoltage radiotherapy machine, most other IGRT techniques add CT imaging capability to a LINAC radiotherapy unit equipped for stereotactic and IMRT use. Because any patient movement between image acquisition and treatment delivery (or during treatment delivery) can introduce error, these IGRT systems use noninvasive immobilization devices and patient position tracking systems. Several manufacturers currently offer IGRT using LINAC technology capable of delivering SRS and radiotherapy, including the Trilogy (Varian Medical Systems, Inc.) and the SynergyS (Elekta, Inc.) equipped with cone-beam CT imaging capability.
 NORMAL TISSUE TOLERANCE IN SRS AND SRT
NORMAL TISSUE TOLERANCE IN SRS AND SRT
Radiosensitivity
Estimating the risks of a proposed treatment plan with various doses is an essential part of treatment planning and dose prescription for SRS and SRT. The ability of normal tissue to tolerate radiation without injury depends on the radiation dose administered, the volume of tissue irradiated, the sensitivity of the tissue affected, history of any prior radiation treatment to the region, and any individual variation in radiation sensitivity between different people. At present, with the exception of patients with known increased radiation sensitivity, such as those with ataxia telangiectasia, there is usually no information available to modify treatment plans for individual differences in radiosensitivity. Prior fractionated radiotherapy appears to have limited effects on the risks of developing parenchymal edema and neurologic sequelae after radiosurgery but has been observed to affect optic nerve tolerance.
Location Effects
Analysis of postradiosurgery injury reactions in AVM patients revealed no difference in the likelihood of postradiosurgery injury imaging changes (increased signal developing in surrounding brain on long relaxation time or T2-weighted images) in different regions of the brain.3,5 Dramatic differences were seen in the rates of developing neurologic sequelae between different regions of the brain (as shown in Fig. 16.2).
FIGURE 16.2. Effect of location on the risk of developing permanent symptomatic neurologic injury following arteriovenous malformation (AVM) radiosurgery.
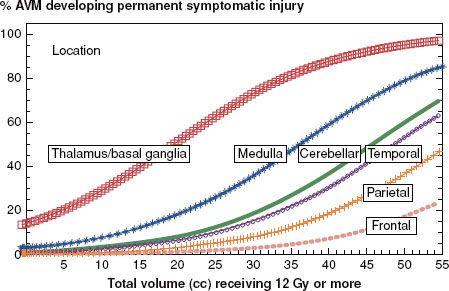
FIGURE 16.3. Phase I Radiation Therapy Oncology Group (RTOG) dose-escalation data for radiosurgery of recurrent brain metastases and glioblastoma fit to logistic dose–response curves. The numbers at each data point indicate the number of patients in each dose/diameter group.6
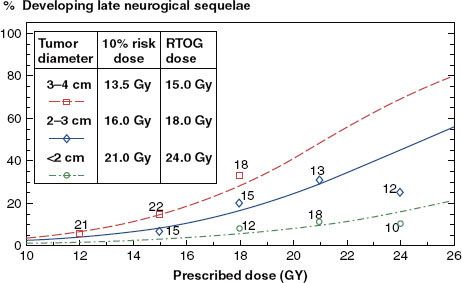
Radiation Therapy Oncology Group (RTOG) Dose-Escalation Studies
The RTOG Radiosurgery Dose-Escalation Study established tolerance doses for radiosurgery of recurrent brain metastases and high-grade gliomas not involving the brainstem.6 They administered radiosurgery to 156 patients with brain metastases or primary brain tumors that recurred or progressed after conventional radiotherapy following a dose-escalation protocol. Starting with initial doses of 18, 15, and 12 Gy for diameters <20, 21 to 30, and 31 to 40 mm, respectively, they escalated prescription doses in 3-Gy intervals until irreversible toxicity was seen in >20% of patients within 3 months. The exception was with tumors <20 mm in diameter where dose-limiting toxicity was not reached and investigators were reluctant to escalate above 24 to 27 Gy. The recommended tolerance doses from that protocol were 24, 18, and 15 Gy for diameters of <20 mm, 21 to 30, and 31 to 40 mm, respectively. The data with longer follow-up beyond 3 months to assess late toxicity were fitted to individual logistic dose–response curves shown in Figure 16.3. These tolerance doses have been widely used as dose guidelines for radiosurgery of malignant tumors, often with interpolation for tumors close to 20 and 30 mm in diameter (e.g., 20 Gy for 18- to 22-mm-diameter and 16.5 Gy for 28- to 32-mm-diameter treatment volumes).
Optic Nerve Tolerance for Radiosurgery
The first analysis of optic nerve tolerance to radiosurgery—a combined Harvard and University of Pittsburgh study of patients with cavernous sinus meningiomas, craniopharyngioma, and pituitary adenomas—recommended 8 Gy as a safe maximum dose limit for the optic nerves/chiasm.7 The lowest optic chiasm dose at which optic neuropathy developed in that study was reported as being 9.7 Gy. Optic nerve/chiasm doses were estimated from isodose distributions overlaid on CT images, unlike the present day when the entire optic system is usually outlined on detailed magnetic resonance (MR) images and maximum doses are assessed from dose–volume histograms. It is highly likely that the true maximum doses to the optic system were higher and laid in portions of the nerve that were poorly visualized.
Stafford et al.8 reported a later analysis of four cases of optic neuropathy occurring out of 215 Mayo Clinic radiosurgery patients with a median dose of 10 Gy to the optic chiasm. One case developed after an optic nerve/chiasm dose of 12.8 Gy with radiosurgery alone, at which the risk level appeared to be approximately 3%. The other cases developed in patients with prior fractionated radiotherapy (7 Gy after 58.8 Gy, 9 Gy after 45 Gy, and two procedures delivering 9 and later 12 Gy to the optic system after 50.4 Gy).
Leber et al.9 analyzed optic nerve injury risks in 50 patients with 24- to 60-month follow-up (median, 40 months) who underwent gamma knife radiosurgery for benign skull-base tumors. Their risks of optic neuropathy were 0% with <10 Gy, 27% with 10 to 15 Gy, and 78% with >15 Gy. They found no cavernous sinus nerve injury with doses of 5 to 30 Gy.
Considering these data and published risks of optic neuropathy for conventional fractionated radiotherapy, α/β ratios in the range of 0 to 1 seem reasonable for estimating fractionated radiotherapy dose equivalents for radiosurgery doses for the optic nerve and probably other cranial nerves.
Tolerance of Other Cranial Nerves
From clinical experience with fractionated conventional radiosurgery and radiosurgery, it appears that special sensory nerves (optic and auditory) are the most radiosensitive, followed by somatic sensory nerves (trigeminal) and finally the motor nerves (cranial nerves II, IV, VI, VII, and IX through XII). After acoustic schwannoma radiosurgery to doses of 12 to 13 Gy, FSR to 18 Gy in three fractions, or SRT to 45 to 50 Gy in 25 to 28 fractions, decreased hearing develops in 30% to 50% of patients, facial numbness in 2% to 3%, and facial weakness in ≤0.5%. Radiosurgery with present techniques for meningiomas involving the cavernous sinus is associated with a risk of trigeminal neuropathy in approximately 3% of patients, with radiation injuries to cranial nerves III, IV, or VI more uncommon.5,10–13
Spinal Cord Tolerance
The tolerance of the spinal cord to SRS or SRT depends on the volume of spinal cord irradiated, the distribution of that radiation (e.g., maximum dose), the dose of radiation previously administered to the spinal cord, and the time interval between initial radiation and retreatment. Spinal cord tolerance to SRS or SRT has not been well defined because of a fortunate paucity of radiation injury reactions in clinical experience so far. Gerszten et al.14 reported on 125 patients who underwent CyberKnife spine radiosurgery (17 benign and 108 metastatic cases). Seventy-eight lesions had previously received external-beam radiotherapy. Treatment volumes varied from 0.3 to 232 mL, with a mean of 27.8 mL. Prescription doses varied between 12 and 20 Gy (mean, 14 Gy) to an 80% isodose treatment volume. Spinal cord volumes receiving >8 Gy varied from 0.0 to 1.7 mL (mean, 0.2 mL). They identified no acute radiation toxicity or new neurologic deficits after a median follow-up of 18 months (range, 9 to 30 months). Benzil et al.15 reported the New York Medical College experience with spine radiosurgery in 31 patients using a Novalis LINAC unit. Two patients who received biologic equivalent doses of >60 Gy developed radiculitis.
 CLINICAL USES OF SRS AND SRT
CLINICAL USES OF SRS AND SRT
Table 16.2 lists the most commonly used indications for radiosurgery, with representative references. Except for functional radiosurgery, there are varied levels of experience with SRT for each of these indications.
TABLE 16.2 COMMON INDICATIONS FOR RADIOSURGERY OR HYPOFRACTIONATED STEREOTACTIC RADIOTHERAPY
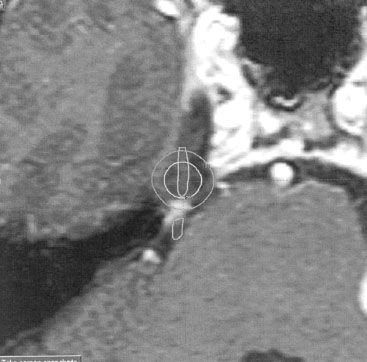
FIGURE 16.4. Typical radiosurgery plan for right trigeminal neuralgia. The right trigeminal nerve is outlined in white. The 30% and 50% isodose volumes from treating a single isocenter with 4-mm-diameter collimators with a gamma unit are shown. A maximum dose of 80 Gy will deliver 40 Gy to the 50% isodose volume and 16 Gy to the 20% volume.
Functional Radiosurgery
The most widely used functional application for radiosurgery is in the management of typical trigeminal neuralgia refractory to medical therapy.3,4,16,17,70 Atypical or constant pain does not respond. Other alternatives for managing typical trigeminal neuralgia are medication, open surgery with microvascular decompression, and rhizotomy procedures using glycerol injection, balloon compression, or radiofrequency injury to the nerve. Typically, 4-mm collimators are used for radiosurgery to a maximum dose of 80 Gy. Response rates reach approximately 85%, typically 1 week to 4 months after the procedure, but can develop as late as 6 months later. Approximately 50% of typical trigeminal neuralgia patients remain pain-free and off medication 5 years following radiosurgery. A typical radiosurgery plan for trigeminal neuralgia is shown in Figure 16.4.
A small destructive lesion in the ventralis intermedius nucleus of the thalamus can be created either invasively with a needle equipped with a radiofrequency generator or noninvasively with radiosurgery using 4-mm-diameter collimators and a maximum dose of 130 Gy to alleviate medically refractory unilateral tremor in patients with essential tremor and/or Parkinson’s disease.1,18–20 Nondestructive management through insertion of a deep brain stimulator is preferred in most patients, but not all patients are acceptable candidates. Limited experiences with radiosurgery of the globus pallidus (pallidotomy) to attempt to alleviate more generalized parkinsonian symptoms have not been as favorable.71–73 There is favorable limited experience with bilateral radiosurgical capsulotomy for managing severe, refractory obsessive-compulsive disorder.74 Radiosurgery to hypothalamic hamartomas may help control refractory gelastic seizures.52,75 The use of radiosurgery as an alternative to extensive surgery in medically refractory mesial temporal lobe epilepsy shows promise and continues to be investigated.55
Vascular Malformations
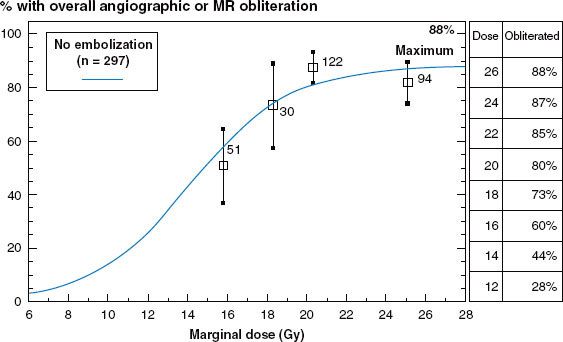
Untreated intracranial AVMs have a bleeding risk of approximately 3% per year, or higher if prior bleeds have occurred.41,44 This results in an average of 1% of untreated AVM patients dying each year from hemorrhage. Management options include observation, surgical resection, embolization, and radiosurgery. Radiosurgery can dramatically reduce the risk of hemorrhage. Radiosurgery obliterates the AVM nidus in approximately 75% of patients within 3 years of the procedure.4,59,76 Individual obliteration rates vary from 50% to 88% depending on marginal dose administered, as shown in the dose–response curve illustrated in Figure 16.5.
Although AVM obliteration rates appear to be optimized with marginal doses of approximately 23 Gy, lower doses are selected for most patients to minimize complications. The risk of neurologic sequelae from radiosurgery averages approximately 3% but varies with treatment volume, dose, and location (Fig. 16.2). The risk of hemorrhage while waiting for complete obliteration to develop seems unaltered.44 All of these risks and benefits of radiosurgery need to be considered together to optimize management of individual AVM patients.
When an AVM nidus fails to completely obliterate by 3 years after radiosurgery, irradiation can be repeated with acceptable morbidity.45 Although some residual radiation injury effect would be expected within the previously irradiated, unobliterated AVM nidus vasculature, retreatment appears to require similar, if not higher, doses to achieve similar rates of complete obliteration as initial radiosurgery.45
Management of large AVMs is presently difficult because radiosurgery may be associated with high complication risks and low obliteration rates. Recent improvements in embolization with liquid glue (ev3, Inc., Plymouth, MN) or polymer can sometimes help reduce the target volume but adds to the total risks of the overall management.77 Another promising approach is staged radiosurgery, in which large AVMs are treated in two or three sections separated by 4- to 6-month intervals to reduce acute toxicity. Whether there is any benefit to fractionating stereotactic irradiation of AVMs is presently unclear.78
Cavernous malformations do not show detectable flow on angiography but nevertheless are vascular lesions with annual hemorrhage risks of 0.5% per year with no prior bleed, 4.5% with one prior hemorrhage, and approximately 32% per year after a history of two or more hemorrhages.77,79–82 Lower pressures in these lesions lead to smaller bleeds than are typically seen with AVM. Repeated bleeds from brainstem cavernous malformations can cause considerable neurologic morbidity. Symptomatic, surgically accessible lesions should be resected. Radiosurgery of brainstem cavernous malformations with a history of two or more prior hemorrhages appears to reduce the risk of subsequent bleeds to approximately 1% per year with acceptable morbidity.76,81,83–85
FIGURE 16.5. Dose response for obliteration of arteriovenous malformation after radiosurgery from 297 patients treated at University of Pittsburgh without embolization.4 MR, magnetic resonance.