Fig. 31.1
Body types, apple and pear
Usually several consultations are necessary before reaching the final decision about the donor site, and as the patient will ultimately have to live with the result, as well as any potential complications, and the scars and defect of the donor area, the decision making must be informed and participatory.
31.3 Flap Options
Although most flaps can be used in both immediate and delayed settings, delayed breast reconstruction requires flaps with greater volume and a larger area of skin for replacing the skin envelope. In contrast, in an immediate situation the patient may need adjuvant treatments or radiotherapy; hence fast and reliable healing of wounds is a higher priority. Postoperative radiotherapy may result in delayed volume loss, scarring or lymphedema. As it is always easier to reduce than to augment later, this can be taken into account by building a larger than necessary breast if radiotherapy is anticipated. Most flaps need refinements at 6–12 months postoperatively, and patients should be informed that it takes months for the scars to soften and the flap to settle into its final position. Secondary scar corrections, «dog ear» removal and free fat grafting can be performed subsequently to optimize outcomes, often under local anaesthesia in an outpatient setting.
31.3.1 Pedicled and Local Flaps
Latissimus Dorsi Myocutaneous Flap
The pedicled latissimus dorsi (LD) flap was first introduced to resurface a thoracic wall defect following a radical mastectomy in 1953 [15]. Since the 1970s, a myocutaneous LD has been the workhorse of breast reconstruction [16, 17]. Even in the era of microsurgery and perforator flaps, it still has a role as a safe, reproducible reconstructive method that can be performed by a single surgeon. Flap failure is rare, vascularization of the muscle, and the skin island is reliable and its pedicle reaches the anterior thoracic wall easily with no need for microanastomosis. The LD flap is ideal for patients with contraindications to microsurgery or if other donor sites cannot provide the necessary volume and an implant is needed. Shoulder problems or massive lymphedema of the arm, occupations or hobbies with high physical demands are relative contraindications to the use of this flap.
The latissimus dorsi muscle is the most extensive muscle in the body and is important in the extension and internal rotation of the upper arm. As a type V muscle in the Mathes Nahai classification [18], it has one dominant vascular pedicle and several secondary paravertebral pedicles. The thoracodorsal pedicle has one artery and one to two veins which arise from axillary vessels via subscapular vessels. The thoracodorsal nerve runs parallel to the vascular pedicle and gives motor function to the muscle. Both vascular pedicle and nerve divide into two main branches at the entrance to the muscle, which enables splitting of the muscle and thus harvesting of a muscle sparing LD (msLD) flap.
The skin island, revealed by a pinch grip, is marked preoperatively either horizontally or obliquely, depending on the body shape and skin type. The patient is positioned in the lateral decubitus position. The skin island and the maximally harvested subcutaneous fat layer are kept intact with the underlying muscle, which is mobilized on its pedicle, proceeding from the mediocaudal to craniolateral direction towards the axilla. The serratus branch of the thoracodorsal pedicle can often be left intact, but posterior attachments are released. Division of the tendinous insertion of the muscle is beneficial, in order to avoid lateral malposition of the reconstructed breast. The thoracodorsal nerve is usually divided and 1–2 cm of its length resected in order to reduce the risk of muscle spasm making the reconstructed breast «jump», which may be disturbing especially if an implant has been placed under the muscle. However, this results in some gradual loss of muscle volume due to muscle atrophy, and some surgeons prefer to leave the nerve intact if no implant is used.
The flap is tunnelled to its new position and fixed temporarily. After donor site closure, the patient is turned into a supine or semi-sitting position. The axilla must be checked, as intercostobrachial nerves and vascular serratus branches may cause kinking of the pedicle, thus risking the flap viability. After securing the pedicle, the flap is positioned and shaped and augmented with an implant, if necessary.
The LD muscle gives good coverage to an implant. An expander prosthesis is an option, if significant additional volume or ptosis of the flap is desired. The use of an expandable implant is also a safe augmentation method in a delayed reconstruction with bad axillary scarring or other risk factors, if flap viability might be compromised by a large volume permanent implant.
In an extended LD myocutaneous flap, the dissection plane is at the level of Scarpa’s fascia, and subcutaneous fat is harvested on top of the muscle, as well as from subscapular and suprailiac regions [19]. Development of free fat grafting makes harvesting of some of the less accessible fat pads, such as that which slides underneath the scapula, of questionable necessity. Nowadays it is common to augment the flap with fat injections to the pectoral and latissimus muscles both during reconstruction and, if needed, in subsequent cosmetic refining operations.
Several authors have proposed muscle sparing LD flap techniques where additional volume from lumbar fat is reinforced with an additional perforator. Although muscle is saved, these techniques are more arbitrary and include a microanastomosis, and the donor site is not hidden as well as in a conventional LD flap [20, 21].
The latissimus dorsi myocutaneous flap as a breast reconstruction method has been criticized for the shape of the reconstructed breast, as well as donor site problems. However, the donor site has an inconspicuous scar on the back, which is easily hidden under the bra and easily forgotten by the patients as she cannot see it daily. Seroma formation in the back donor site wound is common; thus prolonged postoperative suction drainage and later needle aspirations may be necessary. Chronic seroma formation is rare, however.
Scar tension in underlying tissues and numbness of the donor site are common, even if the skin island of the flap has not been large. Shoulder muscle power (adduction, inner rotation, extension) is weakened postoperatively but recovers within a few weeks to permit normal daily activities. Some functional deficit in physical activities may remain, which must be taken into consideration in flap choice for breast reconstruction. Postoperative active rehabilitation has an important role in prevention of these problems [22, 23].
31.3.2 Local Transposition Flaps
Thoracodorsal Transposition Flap (Holmström’s Flap)
The lateral thoracodorsal flap is a fasciocutaneous transposition flap from the posterolateral thoracic wall. It can be harvested as a 6–12 cm wide and up to 22 cm long flap, based medially from the submammary fold. As a traditional local flap with simple flap harvest, it is especially suitable in increasing skin and volume in the inferior pole of the breast. Combined with an implant or free fat grafting, this flap enables even a total breast reconstruction for a morbid patient [24].
Abdominal Advancement Flap
Mobilization of large abdominal skin and subcutaneous flap from the inframammary fold and sliding it up to create the lower pole of the breast are versatile options to create a small breast reconstruction, supplemented with an implant, for a morbid patient. It is also of value in corrective surgery after large volume breast conservation resection or reconstruction [25]. The inframammary fold often drops to a lower level, despite careful fixation, after this technique which may cause asymmetry.
31.3.3 Pedicled Perforator Flaps
Pedicled perforator flaps offer a good choice for partial or small volume breast reconstruction. Since the advent of propeller flaps nearly 30 years ago [26] in reconstructive breast surgery, several different flaps have been introduced [27–29]. In principle free style flaps [30, 31] can be harvested on any perforator of sufficient calibre which supplies redundant tissue. Local flaps allow reconstruction with a number of advantages: a single region operation without the need for microsurgery or a remote donor site, autologous tissue with a more predictable volume than free fat grafting, an excellent colour match and good texture. Suitable tissue sources in the thoracic wall are the back (thoracodorsal artery perforator (TDAP) flap) [32], lateral and anterior thoracic wall (lateral and anterior intercostal artery perforator (LICAP and ICAP) flaps) [33, 34] and contralateral breast (internal mammary perforator (IMAP) flap) [35, 36]. The flap is designed from the area with the greatest amount of redundant tissue, a pinch grip revealing the maximal width of the flap. Flap harvest demands microsurgical skills, careful perforator exploration, appropriate mobilization and good tactical thinking with several alternative plans depending on intraoperative findings.
The perforator pedicle is usually short, which makes positioning of the flap challenging. The flap can be mobilized as a pedicled perforator (TDAP), propeller (LICAP, IMAP), turnover (AICAP) or perforator enhanced transposition flap with a skin bridge (◘ Fig. 31.2).
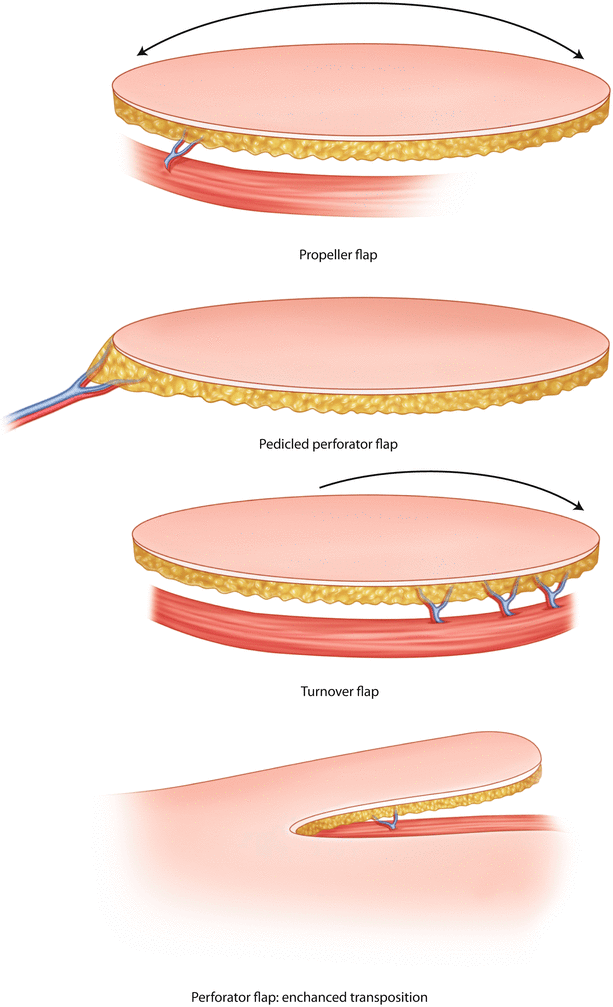

Fig. 31.2
Pedicled perforator flap types
In the lateral thoracic region, perforators and sensate nerves are in close connection to each other, which results in an often sensate flap but may also increase the risk of neuralgia. Thus careful dissection is necessary. Pedicled perforator flaps are more prone to vasospasm than microvascular perforator flaps, which is a relative contraindication for the use of this method in immediate breast reconstructions, especially for morbid patients after neoadjuvant chemotherapy. However, due to minimal donor site problems, these flaps are versatile options for elderly women with shoulder problems or patients with contraindications to microsurgery, e.g. obesity.
Pedicled perforator flaps alone are a good choice in partial reconstructions or shape corrections. In total breast reconstruction, additional volume with free fat grafting or an implant is often needed.
Thoracodorsal Artery Perforator (TDAP) Flap
The thoracodorsal artery perforator (TDAP) flap can be harvested vertically or horizontally. The horizontal skin island is identical to the skin island of the myocutaneous LD flap and is sometimes known as the «LD flap without muscle» [32], except that in the TDAP flap the skin island should be planned approximately two fingerbreadth anteriorly from the lateral edge of the LD muscle in order to include the potential direct cutaneous branch of the thoracodorsal artery to the flap. The flap dissection proceeds from the posteromedial tip of the flap in the suprafascial plane to the level of the scapular tip. The lower edge of the spindle-shaped skin island is incised to reveal the lateral edge of the LD muscle. Thoracodorsal perforators are in a row, usually within 2 cm distance from the muscle edge. Careful dissection proceeds in the supra- or sub-fascial plane to reveal the perforators. If both the thoracodorsal and direct cutaneous perforators are weak, exploration of lateral intercostal perforators is proposed, as they can be stronger in such cases.
Another option is to include the anterior branch of the intramuscular thoracodorsal vessel and a piece of latissimus dorsi muscle, about 1–2 × 3–4 cm in size, as part of the flap and convert the TDAP flap to a muscle sparing LD flap.
The pedicle can be mobilized to the point where posterior and anterior thoracodorsal branches merge to form a common trunk, which gives the pedicle a length of about 4–6 cm and enables a later LD muscle flap to be harvested on its pedicle of the posterior branch. A pedicle length of 15 cm is possible, if mobilization proceeds up to axillary vessels [37–39].
Lateral Intercostal Artery Perforator (LICAP) Flap
The lateral intercostal artery perforator (LICAP) flap is a perforator flap based on the lateral intercostal perforator vessels. Based on this perforator, a long horizontal skin and subcutaneous flap, running towards the back or anteriorly, can be harvested as a propeller flap. In the majority of cases, even very long flaps, «hemithoracic propeller flaps», reaching almost from the spine to sternum of over 40 cm long, can be harvested successfully, if the perforator is strong [33, 34].
Anterior Intercostal Artery Perforator (ICAP) Flap
Anterior intercostal perforators are small and numerous, the largest situating near the midline. The skin flap is designed along the inframammary fold, and the flap can be harvested on a row of several small perforators as a turnover flap. This design is preferable if the inferior pole is low. In cases of medial volume deficit, an AICAP flap, based on a single medial perforator, as a propeller or a perforator enhanced transposition flap gives the needed effect without lowering of the inframammary crease.
Internal Mammary Perforator (IMAP) Flap
The lower pole of the contralateral breast can be used as an internal mammary perforator-based propeller flap to reconstruct a breast, if there is a need for reduction mammoplasty. It is a refreshed old method from the early times of breast cancer surgery [13, 33, 34, 40]. In order to gain success with this method, the quality of breast tissue and skin should be good. Increased cancer risk of the contralateral breast is a contraindication to this method, and the aesthetic result of the healthy breast should not be compromised. Transplantation of the nipple-areola complex is often necessary. The skin overlying the xiphoid process should be preserved if possible, to respect aesthetic units [35, 36, 40, 41].
31.3.4 Microvascular Flaps
Lower Abdominal Flaps
The skin and subcutaneous tissue of the lower abdomen provide an excellent donor site where the tissue quality mimics that of the breast. This elliptical or w-shaped flap between the umbilicus and the suprapubic crease can be raised on several different pedicles according to the technical expertise of the surgeon and the patient’s vascular anatomy.
Although Hartrampf popularized this flap in the 1980s as the pedicled TRAM (transverse myocutaneous rectus abdominis myocutaneous) flap [42], it had already been described as a free flap by Holmström in 1979 [43]. A pedicled TRAM is based on the superior epigastric system, a free TRAM on the deep epigastric vessels, which are the dominant supplier to this skin and subcutaneous fat paddle [44]. A further development came by Koshima [45] who showed that the deep epigastric vessels can be dissected without sacrifice of the rectus abdominis muscle as a perforator flap, and Allen was the first to use this concept in breast reconstruction and called it the DIEP (deep inferior epigastric perforator) flap [46]. He also popularized the idea of Grotting from 1991 to further minimize the donor site morbidity by raising the same flap as a SIEA (superficial inferior epigastric artery) flap on the superficial inferior epigastric vessels [47, 48].
Pedicled TRAM
The pedicled version of the abdominal flap remains a workhorse flap for surgeons and centres that do not have the facilities for free tissue transfer. The flap is raised on the superior epigastric vessels that run through the rectus abdominis muscle. Sacrificing the entire width of the muscle makes dissection safe and easy but can result in abdominal bulging or hernia formation. This risk can be lessened by including only a strip of the muscle, but as the motor nerves run horizontally through the muscle, the preservation of motor function requires more delicate intramuscular dissection. Comparative studies have shown that the donor site defect is similar to that of a muscle sparing free TRAM [49]. In a USA nationwide study of more than 21,000 patients, pedicled TRAM flaps had more pulmonary complications, pneumonia and pulmonary embolisms, but free TRAM patients had more wound complications, longer hospital stay and higher overall cost [50]. In a study by Macadam and colleagues, the pedicled TRAM had more partial failures and fat necrosis than free TRAMs or DIEPS, which is not surprising as the superior epigastric is not the dominant supplier to this flap [44, 51].
Free TRAM
Nahabedian and colleagues classified the free TRAM flap according to the degree of muscle sacrifice:
MS-0, inclusion of the full width of the muscle
MS-,1 preservation of the lateral third of the muscle
MS-2, preservation of both medial and lateral thirds
MS-3, preservation of all of the muscle as in a DIEP flap [52]
Naturally, inclusion of a piece of the whole width of the muscle makes the elevation simpler and safer, but the donor site is equivalent to that of a pedicled TRAM and muscle continuity is lost. Unfortunately comparative studies of the donor site have shown that even if the muscle sparing techniques lessen the abdominal wall donor problems, bulging and hernias still remain a risk [53]. This is probably due to the long fascial opening and splitting of the muscle which damages several segmental nerves, thus denervating the spared muscle.
DIEP
A true benefit for the donor site came from the development of the DIEP flap, as the perforating vessels are dissected through the muscle to reveal the main pedicle within the muscle belly which is carefully dissected free. It is possible to preserve all, or almost all, of the segmental nerves, and thus the remaining muscle will remain functional. Ideally only one perforator, situated in the middle of the flap, is chosen or several perforators in the same row. Studies have indicated that although the flap lives on one perforator alone, less fat necrosis occurs if two to three perforators are included, possibly due to enhanced venous return [54]. However, a rough dissection of several perforators on different sides of the muscle can result in a muscle defect equivalent to that of a muscle sparing (MS) TRAM, and thus it has been difficult to show the donor site benefit of a DIEP over a MS-TRAM. Ideally only a 4–6 cm vertical fascial opening is done to expose the perforator and dissect it through the muscle. If a full length pedicle is needed, one can perform a transverse appendectomy type of incision at a lower level to expose the deep epigastric vessels.
Preoperative assessment of the anatomy of the DIEA perforators helps in planning the operation and in choosing the best perforator. This is best done by performing a CT angiogram of the lower abdomen, but also MRI angiography has been used [55]. This technology not only provides accurate mapping of the vessels but helps to determine which perforator(s) has the shortest intramuscular course and largest calibre. It shows if the deep epigastric has one or two main trunks and if two nearby perforators can be harvested without muscle sacrifice or not. The perforators can of course also be mapped by a handheld Doppler, but this does not reveal their quality or intramuscular course.
A 5-year follow-up study of patients who had undergone the four different methods to raise this abdominal flap showed that DIEP patients had significantly less donor site problems than pedicled TRAM patients, but outcomes did not differ much between TRAM and MS-TRAM techniques [51] (◘ Fig. 31.3).
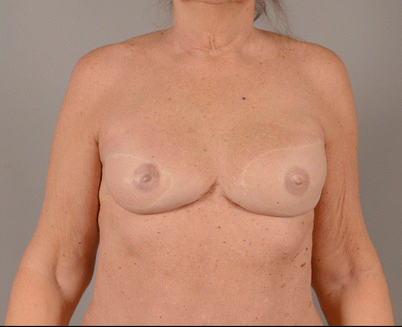

Fig. 31.3
Two flap reconstructions in the same patient, delayed reconstruction with DIEP and immediate reconstruction with TMG
SIEA
Allen presented the idea of raising the lower abdominal flap with the superficial vessels, without muscle sacrifice [48]. These vessels can be exposed by undermining at the level of Scarpa’s fascia in the groin. The vein can be followed to its origin inside the subcutaneous tissue, and the artery runs below it but the origin of the artery is usually 2–3 cm more cranially sited and often merges with the superficial circumflex artery. It is wise to follow both pedicles under the fascia until their entry into the femoral vessels to achieve appropriate diameter and length. The artery is more prone to spasm, and before making the final decision to use this pedicle, it is wise to clamp all DIEA perforators and make sure that the flap is sufficiently vascularized. The SIEA pedicle is usable in only about 10% of the population and is safest when harvested from the axial side only. It is advisable to judge the vascularity preoperatively by either CT or MRI angiography.
Although this technique results in a perfect donor site, it is unfortunately not without problems. The vessel anatomy is variable, and the artery small, and consequently there are higher flap loss and complication rates compared to DIEP or TRAM flaps. Park and colleagues reported their experience of 145 SIEA flaps over an 8-year period, and 80% had arterial problems in the initial operation, with a flap loss rate of 4.8% [56]. A recent Dutch study confirmed that SIEA flaps do have a higher revision and flap loss rate compared to DIEP flaps [57]; however they have a place in bilateral breast reconstructions and in combined lymph node transfer where the benefits outweigh the higher risk.
Thigh Flaps
The upper thigh is a versatile donor site for several flaps suitable to breast reconstruction. The transverse myocutaneous gracilis (TMG, ◘ Fig. 31.3)/transverse upper gracilis (TUG) flap and profunda artery perforator (PAP) flap are the most popular flaps. Also the lateral thigh is an optional donor site in breast reconstruction: anterolateral thigh (ALT) and tensor fasciae latae (TFL) flaps may be used for some patients [58–62].
Transverse Myocutaneous Gracilis (TMG), i.e. Transverse Upper Gracilis (TUG) Flap
Although the gracilis muscle flap and gracilis myocutaneous flap with a vertical skin island have been used in reconstructive surgery for a long time [63], the recent use of a horizontal orientation of the gracilis skin angiosome was only described in the 1990s [64]. At the beginning of this millennium, this flap, with its near invisible donor site, was introduced as a new breast reconstruction method, the transverse upper gracilis flap [65] and soon popularized as the transverse myocutaneous gracilis flap [66].
A spindle-shaped skin island is marked on the proximal thigh, with the upper edge sited along the inguinal-infragluteal crease and the height defined by grasping a tissue fold. The anterior tip is marked medial to the femoral vessels and posterior to the lateral tip of the gluteal fold. The patient is positioned in a supine position with the legs elevated and abducted as for gynaecological surgery. The skin is incised, and the maximal amount of subcutaneous fat is included in the flap and dissection proceeds suprafascially towards both ends of the skin paddle. The fascia is incised above along the adductor longus muscle anteriorly and on the posterior edge of the gracilis muscle posteriorly. No harvest between the skin and gracilis muscle is performed. The subcutaneous fascia is opened on top of the gracilis; the muscle is bluntly dissected free from the surrounding structures until its tendinous insertion, which is divided under palpation control. The secondary pedicle to the SFA is clipped, if possible. The primary pedicles, the medial circumflex femoral vessels, are clipped from side branches and dissected separately until they meet the deep femoral vessels. Additional access lateral to the adductor longus muscle may be used to reveal the deep femoral vessels properly. The motor branch from the obturator nerve is cut and the origin of the muscle divided.
Pedicle vessels are clipped along the femoral vessels and anastomosed either to the internal mammary or thoracodorsal vessels. The breast is shaped, trying to position the skin island in its cranial and medial part and hiding the muscle below the central part of the breast, as muscle atrophy may create a depression in the skin envelope.
The TMG/TUG flap is often the same size as half of the DIEP flap, which makes the method versatile especially in bilateral or immediate breast reconstructions (◘ Fig. 31.4). Even slim patients tend to have subcutaneous fat in the medial thigh, even when the lower abdomen lacks volume. Donor site scars are inconspicuous, the medial part of the scar most frequently descending. Scar tension in the gracilis donor site and posterior thigh numbness are often temporary. Seroma formation and secondary wound healing are relatively common problems, but lower limb lymphedema is rare.