The Ageing of Populations
We have seen that the proportion of elderly citizens increased in developed countries during the 20th century and similar changes are happening even faster in less developed countries. Reasons for this include falling fertility rates and falling death rates at all ages but particularly in infancy and early childhood, owing to improved living standards. The increasing number of very elderly people is, however, partly due to the improvements in medicine in adult life.
Blessing or Curse?
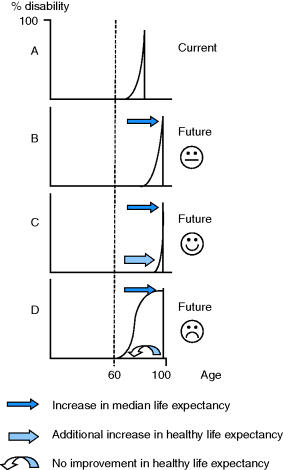
Increasing longevity sounds like a blessing, but only if the extra years are healthy and active. Figure 3.1 shows the possible outcomes (after Tallis 2003). Scenario A is the current situation; people tend to be relatively healthy until their late 60s when chronic disabilities start to accumulate until death. Scenario B shows a situation in which both morbidity and death are postponed. However, the added years may be associated with ‘compression of morbidity’ into the final months of life, the desirable outcome shown in scenario C, or, the worst case scenario, to a prolonged period of disability and dependency, shown in scenario D. One recent estimate gave a 65-year-old English man an average of 10.2 further years of active life followed by 7.3 years of significant disability, and a woman 11.4 years and 8.8 years, respectively (ONS 2010).
The Ageing Process
Cells, tissues and organs all change with age and eventually function begins to deteriorate. Ageing is very complex and poorly understood. There are two major groups of theories:
Wyllie and colleagues have worked on cell death since the 1970s. Whereas an injured cell will swell, burst and release its contents, causing an inflammatory and immune cascade, when a cell dies a normal death, it shrivels up and is consumed by nearby cells so fast it never gets a chance to spill its contents. They named their discovery apoptosis (pronounced apotosis), from the Greek for ‘falling off’, as in leaves from a tree. An array of intracellular and extracellular signals can trigger the cell to enter the process of programmed cell death. Apoptosis occurs from the earliest stages of development (e.g. leading to the separation of fingers), is very active during growth and continues throughout life. Aberrant regulation of apoptosis contributes to cancer, autoimmune disorders, neurodegenerative diseases and systemic viral infection.
It is clear that individual cells can undergo programmed death. There has been debate as to whether multicellular organisms carry programmes to self-destruct (‘a ticking clock’). Ageing as a predetermined, genetically controlled process would be supported by the characteristic lifespans of different organisms. A single gene mutation in the roundworm Caenorhabditis elegans doubles its life expectancy to around 6 weeks. This gene, daf-2, codes for a primitive insulin receptor and regulates an array of other genes which influence longevity. This is part of the mechanism that allows the worm to suspend its development when the environment is harsh. The situation is more complex in man, but the insulin pathway is important in diabetes and cancer which has links with ageing.
Sirtuins are nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases that extend the lifespan of yeast, worms and flies and may be involved in the mechanism by which calorie restriction increases lifespan in animals. There are seven mammalian sirtuins, SIRT1 to SIRT7, which have complex roles in metabolism, homeostasis and the stress response. Different sirtuins are found in the nucleus, mitochondria and cytosol. Major research effort is being targeted at developing sirtuin activators aiming to treat obesity, diabetes and neurodegeneration and even extend lifespan. Resveratrol (a natural compound found in the skin of red grapes and in wine) was thought to be a SIRT-1 activator but the results may have been an artifact. Nevertheless, there is a huge range of Resveratrol supplements for sale on the internet; anti-ageing is big business (see the website of Sirtris Pharma).
Although no genes have been found that double lifespan in man, a study of the genomes of more than 1,000 centenarians, scouring about 300,000 sequence variations for possible links to longevity, has identified around 150 variations in DNA sequence that can predict (with 77% accuracy) whether a person has the genetic wherewithal to live to 100.
Single genetic disorders causing premature ageing (progeria) do occur. These are ‘segmental ageing’ syndromes as they do not result in all the typical features of ageing, but it seems likely that some of the mechanisms involved will be relevant to normal ageing.
- Hutchinson–Gilford syndrome: very rare, sporadic, autosomal dominant causing death around 13 years from MI or stroke. Usually a silent substitution from glycine GGC (A, C, G and T represent the four nucleotide bases of a DNA strand – adenine, cytosine, guanine, thymine) to glycine GGT in codon 608 of the lamin A (LMNA) gene on 1q, which activates a cryptic splice donor site to produce abnormal lamin A. The nuclear fragility of lamin A-deficient cells increases apoptosis and may exhaust stem cell-driven regeneration.
- Werner’s syndrome: rare, autosomal recessive progeria of adulthood caused by mutations in a gene on 8p coding for a member of the RecQ helicase family. Characteristic features include premature greying, skeletal changes and death around 50 years. The Werner protein unwinds, separates and repairs damaged DNA and may be important in maintaining telomeres.
In C. elegans, although genotype determines the mean lifespan of a population, individual longevity has a large stochastic component, with several-fold differences observed even with an isogenic population in a uniform environment.
Wear and tear theories claim that ageing is caused by random accumulated injuries from ultraviolet light, physical damage, toxic by-products of metabolism, etc.
The Ageing Cell
Neurons, renal and myocardial cells do not divide and have to last a lifetime, although the numbers fall. Normal human embryonic fibroblasts have a fixed capacity to divide around 50 times, but those from mature subjects have a reduced capacity for reduplication.
One factor contributing to this may be telomerase inactivation in somatic cells (unlike the germ-line). Telomerase maintains telomeres, regions of repetitive non-coding DNA at the tips of chromosomes that protect them from destruction.
When cells divide, the enzymes that replicate DNA cannot continue all the way to the end of the chromosome so telomeres are gradually used up, becoming shorter every time a cell divides. Eventually they reach a critically short length, the cell cannot divide any more and apoptosis may be triggered. The Nobel Prize for medicine 2009 was awarded for the discovery of how chromosomes are protected by telomeres. Telomerase-deficient mice appear prematurely aged and telomerase reactivation resulted in rejuvenation of some tissues. However, this may carry a cancer risk as telomerase reactivation is a possible explanation for the immortality of malignant cells in culture. A commercial blood test is already available to get your telomere length tested and compared to an age-matched sample to ‘assess your biological age’. This is fascinating, but more work is needed, particularly with regard to the relationship to cancer.
Many other features of cells from aged individuals reflect stochastic events. The biochemistry of the cell is noisy. All cellular processes – transcription, translation, protein folding, every enzymic reaction and all the inbuilt regulatory mechanisms – are subject to random errors. The workings of cells get noisier as they get older, and this might contribute to increasing cellular dysfunction with age. Features of cells from aged individuals include:
- Aneuploidy (variable chromosome numbers).
- Accumulation of somatic mutations of DNA in the cell nucleus and mitochondria.
- Dysregulation of gene expression.
- Misfolded proteins tend to aggregate and ‘gum up the works’, e.g. amyloid. Cells deploy ‘chaperones’ to refold misshapen proteins, but this mechanism may be overwhelmed.
- Lipofuscin pigment granules accumulate in cytoplasm in liver, kidney and muscle (from damaged red cells).
- Oxidative stress and damage as reactive oxygen species are not mopped up by antioxidants and damage key molecules including DNA.
- Less effective cellular pumps (a family of proteins called multidrug resistance proteins) fail to remove toxic products from cells.
- Adducts on macromolecules, e.g. glycation may reflect failure of processes for repair and turnover of macromolecules.
Ageing Tissues
- Loss or dormancy of stem cells: ageing could in part be due to the inability of stem cells to replenish the tissues of an organism with functional differentiated cells needed to maintain them.
- Cross linkages (e.g. disulphide bonds) in collagen and elastin cause increased stiffness in connective tissue especially in skin, elastic laminae of blood vessels, tendons and lens of eye.
- Mitochondrial respiratory-chain function (energy release) is less efficient in skeletal muscle.
Immunity and Ageing
- Thymic involution and attenuated T-cell-mediated immunity lead to reactivation of quiescent infections, such as TB and varicella. There seems to be a decline in delayed-type skin-hypersensitivity reactions to injected antigens (anergy) in many frail aged subjects.
- Autoantibodies occur more frequently (e.g. antiphospholipid antibodies) and are associated with vascular disease, but their significance in older people is uncertain.
- Proliferative disorders of the lymphocyte are very common.
- Malnutrition and diabetes compound these problems.
Unifying Hypothesis
Many of the stochastic cellular events described appear to lead to telomere shortening and many drugs with which we are all familiar lengthen telomeres. Whilst this is too simplistic it is increasingly likely that ageing will eventually be understood in terms of interacting genetic, programmed and random events.
Declining Function
Many physiological parameters decline with age, but the magnitude of the decline is hard to estimate. These figures are almost always based on cross-sectional rather than longitudinal studies, which will include individuals within the elderly cohort who have acquired diseases that may affect function, e.g. renal function will suffer as a result of hypertension or diabetes, or whose sedentary lifestyle in retirement has caused cardiorespiratory fitness to decline through disuse. To be attributable to ageing per se, a phenomenon must be universal, intrinsic and progressive. Watching the London Marathon will reveal many 70 or even 80+ year olds fitter than most people in their 30s and 40s.
Special Features of Illness in Older Patients
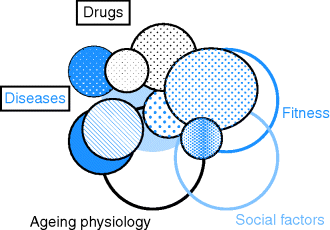
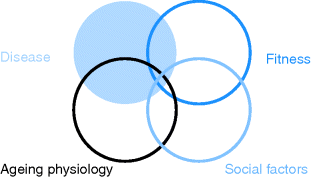
Illness in older people is usually a continuum of conditions found in middle age, but the impact of the illness will be modified by the context of ageing and loss of fitness, and the social situation the person is in (Figure 3.2).
Figure 3.2 Interaction between disease, ageing physiology and the individual’s fitness and social situation.
Background of Ageing
Ageing changes are seen in most organs (go through the body in your mind), including the brain, special senses and peripheral nerves.
Why do Ageing Changes Matter?
- There is increased variability between individuals.
- Okay at rest, significant when stressed (e.g. fasting glucose minimally higher in the elderly, but glucose levels higher after meals).
- Impaired homeostasis results in problems when the environment becomes more challenging. In extreme old age the challenge may be minimal, such as maintaining BP on standing. Some physical signs have different significance, e.g. small pupils, poor upgaze and wasting of small muscles of the hand.
Multiple Pathology and Aetiology
Why do Elderly People often have Several Diseases?
The prevalence of many diseases increases with age (e.g. stroke, PD, AD) so the fact that older people have several diseases may simply reflect this. Some chronic diseases have complications affecting several systems (e.g. diabetes may lead to heart, eye, kidney and nerve problems) or may predispose to other disorders (e.g. infections). Also, a risk factor may predispose the individual to several diseases (e.g. smokers are more likely to have chronic bronchitis, lung cancer, heart disease, strokes, gangrene, macular degeneration and osteoporosis). One problem may also have several causes; e.g. falls are usually multifactorial (previous stroke + cognitive impairment + poor vision + osteoarthritis of the knees, etc.). As discussed later, multiple diseases mean multiple drugs, so the common situation in an older person presents multiple interactions, as shown in Figure 3.3.
Different Risk Factors
It must not be assumed that parameters (e.g. high lipid levels) that constitute a risk factor in the young carry the same risk in the elderly in the absence of positive evidence. An 85-year-old with a cholesterol level of 8 mmol/L presumably has ‘protective’ genes and so the significance of this finding is not the same as in a 40-year-old.
Different Susceptibility to Disease
This is more of a theoretical possibility than a practical consideration. The expected increase in TB as the cohort of people who had survived TB in the pre-drug era became old and frail with poor nutrition and reduced immunity is limited.
Different Differential Diagnoses
Although the range of possible diagnoses may be similar at any age, age is important in determining what is most likely. For example the commonest cause of fits, jaundice and anaemia will be different in the neonate, child, young adult and elderly person.
Altered Response to Disease
Many older people present in exactly the same way as middle-aged people, e.g. crushing central chest pain in an MI. However, this is not always the case, making diagnoses in the frail older person a challenge. There may be:
- Missing symptoms, e.g. lack of pleuritic pain, fever and thirst in pneumonia.
- Missing signs, e.g. lack of neck stiffness in meningitis.
- Intellectual failure (acute or chronic confusion).
- Incontinence (if this is new, why?).
- Immobility (‘off her feet’).
- Instability (falls).
- Iatrogenic disease (see pharmacological treatment).
- Inability to look after oneself (functional decline or, in an analogy to paediatrics, ‘failure to thrive’).
All of these vague and dull-sounding clinical pictures, often labelled ‘social problem’ in the medical records, are almost never due to social problems and could be because of a huge range of serious and treatable conditions – if you look – such as MI, stroke, PD, etc.
Low Expectations
Why do Older People Sometimes Present so Late in Their Illness?
Older people may have poor expectations of the health care system, fuelled by friends and family and sometimes, sadly, by previous experience of health care professionals. ‘What do you expect at your age?’ is a remark familiar to many. The problem may be compounded by lack of medical understanding, so that urinary incontinence and swollen ankles are assumed to be normal in old age.
Social Problems
Old age is a time of loss (family, friends, income, housing, mobility, independence and life itself). There may be practical solutions. However, often what are most appreciated are support and a little of your time to hear about how things were.
Advantages and Disadvantages of ‘Labels’
Be circumspect before labelling people. It is part of the doctor’s job; the label usually helps the patient to understand what is causing their symptoms and helps the clinicians to manage the condition. For example, the Alzheimer’s Society campaigns to get people with dementia recognized and given a diagnosis, as without this they cannot access the help that is available. However, sometimes labels are unhelpful: a 93-year-old woman with impaired glucose tolerance labelled ‘diabetic’ may be refused Christmas cake in her residential home. It is very difficult to shake off an incorrect label and so, if in doubt, remain descriptive, e.g. ‘breathless with shadow on CXR’, pending further investigation.
The Importance of Functional Assessment and Rehabilitation
Expensive and technically successful intervention is of limited value if the patient does not recover the ability to enjoy a worthwhile quality of life. Overall assessment should include a comprehensive list of medical problems and their prioritization in terms of threat to quality and quantity of life (an assessment of cognitive function, evaluation of functional abilities, some idea of the social background [‘ecological niche’] and who is there to do tasks for the patient when they are unable to do them for themselves). It will take an older person longer to recover strength and function after a severe systemic illness – this may be obvious to the reader, but is not always obvious to the patient, family and medical attendant.
Rehabilitation
Rehabilitation is defined as the restoration of the individual to their fullest physical, mental and social capability. It takes several forms:
- Restoration to full activity after a severe illness (e.g. abdominal surgery or MI).
- Restoration of maximum achievable function following a specific impairment (e.g. stroke and fractured hip).
- Facilitating the achievement of as much independence as possible despite continuing impairment (e.g. PD, amputation, stroke and hip disease).
Rehabilitation can take place in a variety of settings (see box below). It is an active process and it is important that the multidisciplinary team, including the patient and carer, share common goals and objectives. If the patient is not progressing as well as anticipated, it is important to look for barriers that may be interfering with the process. These include depression, uncontrolled pain and hidden agendas, e.g. ‘If I improve, I will be a burden to them’.