The incidence of cancer in childhood is quite rare compared with the incidence in adults; approximately 6500 new cases are diagnosed in the United States each year. Nevertheless, cancer remains the second most common cause of death during childhood. The common childhood cancers and their frequency are shown in Table 18.1 . It is important to note that the relative incidence of tumors is not constant through childhood. The median age for neuroblastoma, for instance, is 1–2 years, and the tumor is unusual after 5 years of age. In contrast, the incidence of Hodgkin disease peaks in adolescence and is rare in children younger than 5 years of age.
| Malignant Tumor | Frequency (%) |
|---|---|
| Leukemia (mostly ALL, some AML) | 30 |
| Brain tumors | 19 |
| Lymphomas (Hodgkin and non-Hodgkin) | 13 |
| Neuroblastoma | 8 |
| Kidney (mostly Wilms’ tumor) | 6 |
| Soft tissue sarcoma (rhabdomyosarcoma most common) | 7 |
| Retinoblastoma | 3 |
| Bone | 5 |
| Liver | 1 |
| Other | 8 |
Childhood cancers have provided insights into the biology and treatment of neoplasia in general. The identification of recessive oncogenes, for example, as well as the advantage of multimodal therapy on cure rates were first described in the context of childhood malignancies such as Wilms’ tumor, retinoblastoma, and other solid tumors of childhood.
Several malignancies seen in adults and children are covered in other chapters and will not be reviewed here. Brain tumors and germ cell tumors are discussed in Chapters 8 and 14 , acute leukemias in Chapter 15, and lymphomas in Chapter 16 .
Retinoblastoma
Retinoblastoma is a malignancy of early childhood that arises in the retina. It is often present at birth and is rarely diagnosed later than 6 years of age. The disease is hereditary in 60% of cases. The hereditary type may be transmitted as an autosomal-dominant trait from an affected parent or may occur as a spontaneous mutation. This type of retinoblastoma, which is passed on to 50% of subsequent offspring, is diagnosed earlier than the nonhereditary form and is usually multifocal at presentation. It is associated with the somatic loss of a recessive oncogene, the retinoblastoma ( RB1 ) gene, from chromosome 13. Loss of both RB1 genes leads to the tumor, thus explaining why multiple tumors occur in patients lacking one of the RB1 genes (the first “hit” of the two-hit process has occurred in all the cells). Patients are at high risk for secondary sarcomas, both at previous irradiation sites and elsewhere. A staging system developed by Reese and Ellsworth predicts the likelihood of tumor control and preservation of vision, based on the location of the tumor for disease confined to the orbit (intraocular retinoblastoma).
In contrast to the hereditary form, nonhereditary retinoblastoma is always unilateral and does not have a high risk of second tumors except in the irradiated field. Up to 10% of unilateral tumors may be hereditary, and genetic counseling along with molecular analysis of the germline RB1 genes is necessary for all patients even with unilateral presentation.
Patients with retinoblastoma most commonly present with leukokoria, a whitish reflex from a mass behind the lens. In the United States the disease is usually confined to the globe at diagnosis, although it may grow along the optic tract or extend through the globe and invade bone. Microscopically, retinoblastoma cells have hyperchromatic nuclei with scanty cytoplasm. The vast majority of patients with retinoblastoma are cured with a combination of surgery and/or radiation therapy. Chemotherapy is used only for the very rare patient with extraocular disease or distant metastases.
The size and extent of retinoblastoma can be measured by many means including direct ophthalmoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and sonography. Fluorescein angiography (not illustrated) will detect small vascular lesions. It is performed by intravenous injection of a fluorane dye and direct photography of the retina illuminated by an ultraviolet light.
Wilms’ Tumor
Wilms’ tumor is the most common tumor of the kidney in childhood; other rarer, malignant renal tumors of childhood include clear cell sarcoma and rhabdoid tumor. The median age at diagnosis of Wilms’ tumor is between 2 and 3 years. The tumor is uncommon after 8 years of age. The classic histologic appearance of Wilms’ tumor is triphasic, comprising blastemal, epithelial (tubules and glomeruloid structures), and mesenchymal elements, although many tumors exhibit only one or two of these elements. The presence of anaplasia is associated with a poorer prognosis.
Most patients with Wilms’ tumor present with an abdominal mass, usually first palpated by the parents. Less common presentations include hematuria and hypertension; systemic symptoms are rare. Bilateral tumors are found at presentation in 4% to 7% of cases. In 5% of cases Wilms’ tumor is associated with other abnormalities of the genitourinary (GU) system and, more rarely, with hemihypertrophy, the Beckwith-Wiedemann syndrome (macroglossia, somatic gigantism, abdominal wall defects, and hypoglycemia), Drash syndrome (pseudohermaphroditism and nephropathy), and aniridia. The latter syndrome led to the discovery of an association in some patients between Wilms’ tumor and loss of the WT1 gene on the long arm of chromosome 11. This gene is a zinc-finger DNA-binding protein that is expressed in the developing kidney.
The grouping system developed by the National Wilms’ Tumor Study is shown in Figure 18.7 . The workup for Wilms’ tumor includes urinalysis, chest radiography, and abdominal and chest CT scan. The lung is the most common site of metastases. MRI of the abdomen may also be useful and may substitute for abdominal CT. MRI may help differentiate nephrogenic rests from bilateral Wilms’ tumor. Either MRI or ultrasonography of the abdomen should be done preoperatively to visualize tumor within the inferior vena cava (IVC).

Neuroblastoma
Neuroblastoma is the most common extracranial solid tumor in childhood and the most common cancer in infants. The median age at diagnosis is 2 years, although neuroblastoma occasionally occurs in adults. The tumor, originating in neural crest cells, can occur anywhere along the sympathetic nerve chain. By far the most common location for a primary tumor is the adrenal gland. In its most primitive form, the histology of neuroblastoma is marked by poorly differentiated small, round blue cells. At an intermediate stage of maturation, there is differentiation toward ganglion cells. Tumors composed of a mixture of neuroblasts and mature ganglion cells are classified as ganglioneuroblastomas. At the most differentiated end of the spectrum is ganglioneuroma, a benign tumor composed entirely of mature ganglion cells, neuritis, and Schwann cells.
The International Neuroblastoma Staging System (INSS) is shown in Table 18.2 .
| Stage | Definition |
|---|---|
| 1 | Localized tumor with complete gross excision, with or without microscopic residual disease; representative ipsilateral nonadherent * lymph nodes negative for tumor microscopically |
| 2A | Localized tumor with incomplete gross excision; representative ipsilateral nonadherent lymph nodes negative for tumor microscopically |
| 2B | Localized tumor with or without complete gross excision, with ipsilateral, nonadherent lymph nodes positive for tumor. Enlarged contralateral lymph nodes must be negative microscopically. |
| 3 |
|
| 4 | Any primary tumor with dissemination to distant lymph nodes, bone marrow, liver, skin, and/or other organs (except as defined for stage 4S) |
| 4S | Localized primary tumor (as defined for stages 1, 2A, or 2B), with dissemination limited to skin, liver, and/or bone marrow ‡ (limited to infants <1 year of age) |
* Lymph nodes attached to and removed with the primary tumor.
† The midline is defined as the vertebral column. Tumors originating on one side and crossing the midline must infiltrate to or beyond the opposite side of the vertebral column.
‡ Marrow involvement in stage 4S should be minimal (i.e., <10% of total nucleated cells identified as malignant on bone marrow biopsy or on marrow aspirate). More extensive marrow involvement would be considered to be stage 4. The MIBG scan, if performed, should be negative in the marrow.
Stage 4S neuroblastoma is an unusual malignancy that has a high likelihood of spontaneous resolution despite the existence of extensive, generalized metastases at the time of presentation. The most common fatal complication in stage 4S disease is respiratory compromise secondary to massive enlargement of the liver. For other patients with neuroblastoma, the prognosis overall is stage- and age-related, with a markedly improved survival rate for patients who present before the age of 1 year.
The presenting signs and symptoms of neuroblastoma depend on the location of the primary tumor. An abdominal mass associated with an adrenal tumor is the most common presentation. Paraspinal tumors may present with cord compression, and thoracic tumors are occasionally associated with Horner syndrome. Patients with metastatic disease frequently present with proptosis and periorbital ecchymosis. Metastases occur to lymph nodes, bone, bone marrow, liver, and skin. Patients frequently show systemic symptoms of irritability and poor food intake, probably occasioned by pain from extensive bone involvement. Urinary excretion of catecholamines is increased in 85% to 90% of patients with neuroblastoma; in fact, the diagnosis can be made on the basis of increased urinary catecholamines associated with typical neuroblastoma cells in the bone marrow. Workup should also include a bone scan and MR or CT imaging of the primary; many centers are doing MIBG scanning, utilizing the tumor uptake of the radiolabeled catecholamine precursor meta-iodobenzylguanidine. Rarely, patients present with diarrhea from secretion of vasoactive intestinal polypeptide or with the syndrome of opsoclonus-myoclonus; the latter syndrome may be an autoimmune phenomenon and may not regress even with successful treatment of the tumor.
Neuroblastoma is one of the first tumors noted in some cases to involve amplification of a dominant oncogene. Cytogenetic studies of cell lines and fresh tumor specimens may show double minutes or homogeneous staining regions representing multiple copies of the MYCN oncogene. Amplification of this gene conveys a less favorable prognosis. Several other biologic variables predict outcome in neuroblastoma. The International Neuroblastoma Risk Groups Committee is attempting to define patient subsets based on these variables.
Hepatic Tumors
The most common primary malignant tumors of the liver in childhood are hepatoblastoma and hepatoma (hepatocellular carcinoma). (See also text and illustrations on “Hepatoma” in Chapter 7 .) The distinguishing clinical characteristics of hepatoblastoma and hepatoma are shown in Table 18.3 .
| Characteristic | Hepatoblastoma | Hepatoma |
|---|---|---|
| Age | 0–3 years | 5–18+ years |
| Previous liver disease | Uncommon | Common |
| Pain | Uncommon | Common |
| Jaundice | Uncommon | ¼ of cases |
| Elevated α-fetoprotein | 2/3 of cases | ½ of cases |
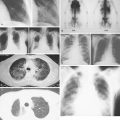
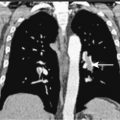
Hepatoblastoma is a rare, embryonal malignant neoplasm that typically presents in infancy, showing a predilection for males. It may be associated with a variety of congenital anomalies and with the syndrome of familial polyposis coli (FPC). Children with hepatoblastoma and FPC may exhibit congenital hypertrophy of the retinal pigment epithelium (CHRPE), which is sometimes seen with FPC. There is an increased incidence of hepatoblastoma in low-birth-weight infants; the cause for this association is not known. Most children with hepatoblastoma present with an asymptomatic abdominal mass, which grossly appears tan and lobulated. Workup includes measurement of α-fetoprotein, which is elevated in nearly all patients. CT scan of the lung, the most common site of metastasis, is part of patient workup, as well as abdominal MRI, ultrasonography, and, finally, angiography of the liver if the potential for resection is unclear. Complete resection of the primary tumor is of utmost importance for cure in hepatoblastoma. Adjuvant chemotherapy improves outcome in completely resected cases, and neoadjuvant chemotherapy may convert some patients’ tumors from being unresectable to resectable; this approach has considerably increased the cure rate in hepatoblastoma.
Rhabdomyosarcoma
Rhabdomyosarcoma, a malignancy that differentiates toward striated muscle cells, is the most common soft tissue sarcoma of childhood. (See also text and illustrations on “Rhabdomyosarcoma” in Chapter 12 .) Pathologic sections occasionally show cross-striations, but their presence is not necessary to establish the diagnosis. Antibodies against muscle-specific proteins (especially myogenin and Myod1) and electron microscopy showing bundles of actin and myosin filaments can be extremely helpful in distinguishing rhabdomyosarcoma from other tumors. Two histologic patterns have been identified: embryonal and alveolar. Cytogenetics is an important tool in the diagnosis of rhabdomyosarcoma. The vast majority of tumors with alveolar characteristics have a translocation between the FKHR (now FOXO1 ) gene (one of the forkhead transcription factor genes) on chromosome 13 and the PAX3 gene on chromosome 2 (or, less commonly, the PAX7 gene on chromosome 1).
The median age at presentation for childhood rhabdomyosarcoma is 2–3 years for GU tumors and 6 years for tumors of the head and neck (see Table 18.4 ). Patients with rhabdomyosarcoma are assigned both a stage and a group. The stage is determined by factors at presentation. The system used is a TNM system but also incorporates the site of the tumor, since this is highly prognostic. Grouping is a surgical staging system and helps determine local control methods and dosing of radiation therapy. It is also prognostic when added to stage (see Table 18.5 and Figure 18.25 ), although in recent years there has been greater use of a TNM system. Proper staging requires MRI of the primary, CT of the lungs, and bone marrow aspirates and biopsies. As with other childhood sarcomas, FDG-PET imaging is being used increasingly for staging and post-treatment evaluation.
| Location | Relative Frequency (%) | Median Age (years) | Histology |
|---|---|---|---|
| Head and neck | 40 | 6 | Embryonal > alveolar |
| Genitourinary | 20 | 2–3 | Embryonal |
| Extremity, trunk | 30 | 12–20 | Alveolar > embryonal |
| Stage | Sites | Tumor (T) * | Size | Node (N) † | Metastasis (M) ‡ | |
|---|---|---|---|---|---|---|
| I |
| T 1 or T 2 | a or b | N 0 , N 1 , or b | N x | M 0 |
| II | Bladder/prostate, extremity, cranial, parameningeal, other (includes trunk, retroperitoneum, etc.) | T 1 or T 2 | a | N 0 or N x | M 0 | |
| III | Bladder/prostate, extremity, cranial parameningeal, other (includes trunk, retroperitoneum, etc.) | T 1 or T 2 | ab | N 1 N 0 , N 1 , or N x | M 0 | |
| IV | All | T 1 or T 2 | a or b | N 0 or N 1 | M 1 | |
* T 1 , confined to anatomic site of origin; T 2 , extension and/or fixative to surrounding tissue; a, ≤ 5 cm in diameter in size; b, >5 cm in diameter in size.
† Regional nodes: N 0 , regional nodes not clinically involved; N 1 , regional nodes clinically involved by neoplasm; N x , clinical status of regional nodes unknown.
‡ Metastasis: M 0 , no distant metastasis; M 1 , metastasis present.

Surgery, radiation therapy, and chemotherapy are all important for long-term survival. Prognosis depends on the site of origin of the tumor and its histology: orbital, paratesticular, and most GU primary tumors have a more favorable prognosis than those of the extremities, trunk, and head and neck with extensive bone destruction; the alveolar histologic variant carries a less favorable prognosis than the embryonal variant.
The Ewing Family of Tumors
EWING SARCOMA AND PERIPHERAL PRIMITIVE NEUROECTODERMAL TUMOR OF BONE AND SOFT TISSUE
Ewing sarcoma is the second most common malignant bone tumor in children and adolescents. (See also text and illustrations on “Ewing Sarcoma” in Chapter 12 .) Although its cell of origin is unclear, its histology is marked by small, round blue cells, classically often containing periodic acid–Schiff-positive material. Ewing sarcoma of bone and soft tissue has a continuum of differentiation, with the most differentiated form frequently termed primitive neuroectodermal tumor (PNET). The pathogenesis and cytogenetics of PNET are clearly different from the group of central nervous system tumors (central PNET) that unfortunately carry the same name. PNETs exhibit neuroectodermal differentiation as demonstrated on electron microscopy or by positivity with neuronal markers such as neuron-specific enolase. The clinical importance of this variation in differentiation is unclear. PNET and Ewing sarcoma cells both stain positively to antibodies to CD99, the protein product of the MIC2 (now CD99 ) gene. Ewing sarcoma and PNET have a high incidence of a clonal abnormality, a translocation between chromosomes 11 and 22. This translocation creates a novel chimeric protein consisting of parts of two genes: FLI1 (an ETS-like oncogene) and EWS (now EWSR1 ), a gene encoding an RNA-binding protein. This information can often be obtained by needle aspiration, using polymerase chain reaction or fluorescence in situ hybridization techniques. These tumors have a peak occurrence in the early second decade but can occur in patients as young as infants or as old as 30 years of age. The disease is extremely rare in blacks and Asians.
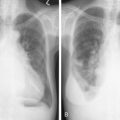
For patients with bone primaries, the most common symptom is a painful mass. For both soft tissue and bone primaries, fever may be a symptom at presentation in about one third of patients and occurs more commonly with large tumors or tumors with metastases. The most common bone site is the pelvis, followed closely by the femur, although the tumor can occur in virtually any bone. When a long bone is the site of the primary tumor, the midshaft of the femur is the most commonly involved area. Soft tissues in nearly every region of the body can develop PNETs, including the extremities, pelvis, or retroperitoneum. Most common sites include the paraspinal and thoracopulmonary regions. The paraspinal masses often impinge on the spinal cord. The thoracopulmonary tumor is commonly referred to as the Askin tumor of the chest wall.
The workup of a patient with Ewing sarcoma/PNET should include plain films and MR scanning of the primary tumor. In addition, CT scan of the lungs, bone scan, bone marrow aspiration and biopsy, and determination of lactate dehydrogenase concentration are part of the patient workup. There is also an increasing role for FDG-PET imaging, both for staging and post-treatment evaluation. The most common site of metastases is the lung, followed by bone and bone marrow. Poor prognostic factors for patients with bone primaries, in order of importance, are the presence of metastases, large size of the primary tumor, and location of the primary. Pelvic involvement carries a worse prognosis than involvement of the femur or humerus, which in turn has a worse prognosis than involvement distal to the knee and elbow. Treatment includes systemic chemotherapy for control of micrometatastic disease, which is present in more than 90% of patients, as indicated by the cure rate achieved with amputation alone. Radiation therapy can control the primary lesion in the majority of patients; it is unclear whether or not surgical excision of the primary tumor improves local control or overall disease-free survival.
Osteosarcoma
Osteosarcoma (osteogenic sarcoma) is the most common malignant tumor of bone in children and adolescents. (See also text and illustrations on “Osteosarcoma” in Chapter 12 .) Derived from primitive mesenchymal bone-forming cells, osteosarcoma is defined histologically by malignant sarcomatous cells that form osteoid. The tumor most commonly arises in the second decade of life, with peak occurrence during the adolescent growth spurt. Most adolescents present with pain, often in conjunction with a soft tissue mass. Osteosarcoma is primarily a disease that affects the metaphysis of long bones. The distal femur is the most common primary site, followed by the proximal tibia and the proximal humerus.
Workup of a patient with presumed osteosarcoma should include plain films and MR scan of the primary lesion, as well as CT scan of the lung, bone scan, and determination of lactate dehydrogenase concentration. Metastases at the time of presentation are associated with a poor prognosis. Successful treatment of patients with nonmetastatic osteosarcoma requires complete surgical excision of the primary tumor and adjuvant chemotherapy. It is not clear at present whether preoperative chemotherapy improves the overall survival rate.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree