Fig. 21.1
Distribution of primary sites of rhabdomyosarcoma and survival according to tumor location
Different tumor sites may be associated to different RMS subtype: botryoid histology is seen commonly in the mucosa of the female genital tract and in the head and neck region of young children, while alveolar RMS is common in the extremities of adolescents.
Regional lymph node dissemination is present in around 20 % of cases (it is higher in alveolar RMS, in adolescents, and in tumor of the extremities). Distant metastasis is present in 15–25 % of newly diagnosed patients, lung being the most common site of hematogenous metastasis (40–50 %), followed by bone marrow (10–20 %) and bone (10 %).
Diagnosis, Risk Stratification, and Prognosis
Table 21.1 describes initial diagnostic work-up and information needed before proceeding to treatment. Ultrasonogram is often the first instrumental assessment to be used. Computed tomography (CT) scan or magnetic resonance imaging (MRI) of the primary site is mandatory for the local extension assessment before any treatment (MRI could be considered superior in defining soft tissue extension). Distant assessment requires chest CT scan, Technetium bone scan, abdominal ultrasound, and bone marrow aspiration plus trephine biopsy, to identify lung, bone, abdominal, and bone marrow dissemination, respectively. Special sites may require particular evaluations, i.e., cerebrospinal fluid cytology in parameningeal RMS, to assess meningeal dissemination; regional lymph node biopsy in extremity RMS; retroperitoneal lymph node sampling in paratesticular RMS older than 10 years [7–9].
Table 21.1
Approach to patients with rhabdomyosarcoma before initiating treatment
Collect data at diagnosis | Patient | Physical exam | Imaging studies |
Age | Lymph node (special sites: neurological exam to detect cranial nerve palsy in parameningeal RMS) | Local imaging (MRI/CT) | |
Nutritional status | Distant metastases stage | ||
Tumor | Chest CT | ||
Size (< or ≥5 cm) | Bone scan | ||
Site (see below) | Other studies | ||
Bone marrow biopsies | |||
Tumor site | Favorable | Orbit | |
Head-neck non-parameningeal | |||
Genitourinary non-bladder/prostate | |||
Unfavorable | Parameningeal | ||
Extremities | |||
Other sites: trunk, chest, abdominal wall, etc. | |||
TNM classification | T1 and T2 based on local invasiveness | N0/N1 and M0/M1: absence or presence of nodal and distant involvement | |
A or B, i.e., less or more than 5 cm | |||
Assess regional lymph nodes | Physical exam | All tumors | |
CT/MRI | Important for pelvic and extremity tumors | ||
Sentinel node biopsy | Consider for extremity tumors | ||
Retroperitoneal sampling | Consider for paratesticular tumors | ||
Assess resectability | Resectable | Conservative complete excision with negative margins | |
Unresectable | Biopsy only | ||
Extent of resection | IRS grouping | ||
Group I | Completely excised tumors with negative microscopic margins | ||
Group II | Grossly resected tumors with microscopic residual disease and/or regional lymph nodal spread | ||
Group III | Gross residual disease after incomplete resection or biopsy | ||
Group IV | Metastases at onset | ||
Histology | Favorable | Embryonal, spindle cell, botryoid | |
Unfavorable | Alveolar | ||
Before proceeding to treatment, we should have the following information | 1. Imaging of primary tumor (essential for radiotherapy planning) | ||
2. Full surgical report | |||
3. Pathology data (histology and margins) | |||
4. Lymph node assessment if needed | |||
5. Metastatic work-up done (chest CT, bone scan, bilateral bone marrow biopsies) | |||
6. Stage | |||
The initial biopsy (incisional biopsy or tru-cut) has the aim to define the histological diagnosis and should be the initial surgical procedure in all patients, also when a subsequent primary excision is planned. Initial biopsy must be carefully planned by experienced surgeons, taking into account the possible subsequent definitive surgery, which must include the scar and the biopsy tract (for example, in RMS of the extremities, the incision must be longitudinal to the limb and not traverse multiple compartment; very careful hemostasis must be ensured to avoid postsurgical hematoma and drains).
The prognosis of RMS depends on multiple factors, including age, primary tumor site and size, lymph node involvement, histology, surgical resection, and distant metastasis. In the past 30 years, the cure rates for RMS have improved dramatically from 25 to 30 % (before the modern chemotherapy-era) to approximately 70 %, due to the development of multidisciplinary and risk-adapted treatment approaches conducted by International cooperative groups. Of course, not all patients with RMS fare well with modern therapies. Patients with alveolar histology continue to have less than optimal outcome. Most patients with distant metastasis do not achieve long-term cure and may benefit of more intensive treatment and are candidates for experimental treatment with novel agents [10].
With the identification of different prognostic factors [11–16], risk assessment has now become more complex, but also more accurate. The approaches of Children Oncology Group (COG) (Table 21.2) and European pediatric Soft Tissue Sarcoma Study Group (EpSSG) (Table 21.3) for risk stratification use similar principles but with different approaches. The EpSSG, for example, identifies low, standard, high, and very high-risk groups (with eight subgroups) for localized RMS, plus the group of metastatic RMS cases; the EpSSG high-risk group grossly corresponds to the COG intermediate-risk group.
Table 21.2
Children Oncology Group (COG) risk stratification
Risk | Estimated 5-year EFS (%) | Description | Current treatment |
|---|---|---|---|
Low risk | 90 | Nonmetastatic embryonal tumors | Subset 1: VAC × 4 cycles followed by VA for a total of 24 weeks |
Except intermediate risk | Subset 2: VAC | ||
Intermediate risk | 65–73 | Nonmetastatic embryonal tumors in unfavorable locations (stage 2 or 3) with incomplete resection (clinical group III) and All nonmetastatic alveolar | VAC |
ARST0531 study randomizes patients between VAC and VAC + VI | |||
High risk | <30 | All metastatic | ARST0431 backbone (benefit with multiagent chemotherapy with interval compression—dose-density: VAC + VDC + VI + IE) |
Table 21.3
European pediatric Soft Tissue Sarcoma Study Group (EpSSG) RMS 2005 risk stratification
Risk group | Subgroup | Pathology | Postsurgical stage | Site | Node stage | Size and age | therapy |
|---|---|---|---|---|---|---|---|
Low risk | A | Favorable | I | Any | N0 | Favorable | VA |
standard risk | B | Favorable | I | Any | N0 | Unfavorable | IVA + VA or IVA ± XRT |
C | Favorable | II, III | Favorable | N0 | Any | ||
D | Favorable | II, III | Unfavorable | N0 | Favorable | ||
High risk | E | Favorable | II, III | Unfavorable | N0 | Unfavorable | First random: IVA + XRT vs. IVADo + XRT |
F | Favorable | II, III | Any | N1 | Any | Second random: maintenancea vs. stop therapy | |
G | Unfavorable | I, II, III | Any | N0 | Any | ||
Very high risk | H | Unfavorable | I, II, III | Any | N1 | Any | IVADo + XRT + maintenance |
Treatment
RMS is a rare tumor and its treatment is necessarily multidisciplinary and complex. The overall multimodality treatment strategy involves surgery, radiotherapy, and chemotherapy, and it is important that the optimal intensity and timing of these treatment modalities should be planned with regard to the patients’ risk stratification and late effects of treatment. In particular, radiotherapy needs to be used with caution in children, given the important sequelae of these treatments. For example, survivors after parameningeal RMS (requiring full doses and large volume of radiotherapy) have a high risk of facial growth retardation (bone and soft tissue hypoplasia, facial asymmetry), but also dental abnormalities, neuroendocrine dysfunctions (growth hormone deficiency, hypothyroidism), visual problems, hearing loss.
Chemotherapy
The VAC regimen (vincristine, actinomycin-D, cyclophosphamide, given at 1.2 mg/m2/cycle) is the gold standard for chemotherapy for RMS in North America. On the other hand, the standard in Europe is considered the IVA regimen (ifosfamide, given at 6 g/m2/cycle, vincristine, actinomycin-D), which differs only in the choice of alkylating agent—probably producing a slightly different pattern of hematological, renal, and gonadal toxicity. The Intergroup Rhabdomyosarcoma Study (IRS)-IV study found no differences in survival rates in a randomized comparison between VAC and IVA [17]. The duration of treatment is currently 6–12 months according to different protocols.
As mentioned above, the risk stratification as adopted by the collaborative groups directs the treatment direction. In the COG most recent low-risk RMS trial (ARST0331), patients with subset I (Table 21.2) had an excellent outcome (2 year EFS, 88 %; OS, 98 %) with short therapy duration (22 weeks) and a low cumulative dose (4.8 g/m2) of cyclophosphamide. On the other hand, subset 2 had a 3-year EFS of 66 % using low dose of cyclophosphamide [18]. This group had better outcome on the previous protocols with standard doses of cyclophosphamide.
The COG intermediate-risk/EpSSG high-risk RMS category is currently treated with the standard VAC or IVA regimen. Adding other agents to these regimens by collaborative groups did not result in significant impact to survival, so far. Among other drugs that were tested, camptothecin derivatives (topoisomerase I inhibitors) showed the best outlook. Addition of topotecan to the standard VAC regimen for intermediate-risk RMS failed to show benefit [19]. Nevertheless, the more promising drug, irinotecan, combined with vincristine is being evaluated in combination with the VAC regimen in the same population of patients.
Doxorubicin is an effective drug in RMS, but the role of anthracyclines as part of a multidrug regimen remains somewhat controversial. For that reason doxorubicin is being evaluated in the current EpSSG trial (Table 21.3). It was also added to the COG high-risk RMS trial ARST0431 which is currently used as a backbone for future trials in high-risk patients [10]. Of note, this regimen incorporated irinotecan/vincristine and ifosfamide/etoposide and used an approach of “dose-compression”—increase of chemotherapy dose intensity and dose density by administering chemotherapy cycles at 2-week interval instead of the usual 3-week interval—similar to that used successfully for Ewing sarcoma [20]. In fact, the prognosis of metastatic RMS remains poor and their management is the subject of ongoing trials. The limited pool of these patients makes it difficult to conduct randomized trials to answer critical questions, but it is agreed that treatment intensification is warranted for this group of patients. High-dose, myeloablative chemotherapy, followed by autologous stem cell rescue, has been variously attempted in metastatic RMS patients. Weigel et al. reviewed 389 patients reported in the literature who underwent myeloablative chemotherapy for metastatic or recurrent RMS [21] and found the outcome much the same as in reports on metastatic patients given conventional therapy [22, 23]. This approach remains experimental and should not be considered as a standard approach for patients with high-risk RMS.
Finally, a potentially interesting option is that of maintenance therapy (metronomic therapy, i.e., regular, frequent administration of low doses of drug with the aim to achieve an anti-angiogenic effect). Currently, the approach of a 6-month maintenance therapy comprising a combination of vinorelbine and low-dose oral cyclophosphamide is under investigation in the EpSSG RMS trial for high-risk patients [24] (Table 21.3).
Possible complications of chemotherapy should be always taken into account. The VAC regimen has serious toxicity that might be exploited in malnourished and very young population. Some of these toxicities are tolerable, including neuropathy that is commonly observed after weekly administration of vincristine. Neutropenia is often observed but is less expected if lower doses of cyclophosphamide are used [25]. A very serious complication is hepatopathy, in the form of veno-occlusive disease (VOD) [26]. This potentially fatal complication is observed mainly in children less than 3 years of age and warrants careful dosing of vincristine and actinomycin-D in this group. Acute and late cardiotoxicity is a known complication of doxorubicin.
Radiotherapy
Radiotherapy is the mainstay of treatment in RMS, since local progression or relapse continues to represent the major cause of treatment failure. Radiotherapy is generally delivered to the pretreatment tumor volume with doses generally ranging between 40 and 55 Gy [17, 27–35]. Three-dimensional conformal radiotherapy—if available—may reduce the long-term toxicity by avoiding unnecessary exposure to vital structures. Similarly, intensity-modulated radiotherapy (IMRT), proton radiotherapy, and interstitial brachytherapy may provide adequate local control with better delineation of the treatment area, and hence, decreased toxicity.
Various issues on radiotherapy in RMS remain to be clarified: should all patients with RMS receive radiotherapy? Can the dose of treatment be modified based on response to treatment and delayed surgical resection? Is it possible to reduce the volume of radiotherapy based on new tumor volume following treatment with chemotherapy and/or surgery?
As for the first question, there is a general consensus that radiotherapy may be omitted in IRS group I patients (initial complete resection) with favorable histology, whereas it must be always required for alveolar histotypes. COG protocols suggest radiotherapy for all RMS patients except for IRS group I embryonal RMS [30], while in European groups it is more debated the indication of radiotherapy in IRS group III patients (patients with initially unresected tumor) after delayed complete surgery or after complete remission to initial chemotherapy, for those tumors arising in particularly favorable sites (i.e., orbit or vagina), especially for young children.
As for the second question, COG recently published its experience with dose reduction (up to 36 Gy) in patients with low-risk embryonal RMS, based on completeness of surgical resection of the primary tumor. Local control was adequate when cyclophosphamide was given (patients treated with VAC regimen), but the analysis suggested that radiotherapy dose reduction should be avoided in patients treated with two drugs only (VA) [34].
The third question remains unanswered: it has been suggested that volume reduction (from the pre-chemotherapy to the post-chemotherapy volume) may be potentially safe (but not for parameningeal cases) [35], but to date the standard treatment volume should remain the pre-chemotherapy one, except for very selected situations (e.g., large pelvic or chest wall tumors where pretreatment volume radiation exposes normal structures to untolerable doses of radiation).
Surgery
Surgery for RMS has evolved over the years from the primary treatment modality (prior to the introduction of effective antineoplastic agents) to one component of a multidisciplinary approach, and from an aggressive surgery to a more conservative organ-sparing procedures, to the point that chemotherapy and radiotherapy may permit in some cases to cure the disease without any surgery (i.e., patients with parameningeal RMS).
Surgery with risk of anatomic or functional impairment is not recommended as first surgical approach and should be considered only as salvage treatment, after the failure of other procedures (special circumstances must be considered, however, e.g., a lower extremity RMS in a toddler, the choice between amputation and radiotherapy, with its long-term effects on limb growth, may pose a difficult dilemma). Tumors considered unresectable at diagnosis can be conservatively and completely resected in a large percentage of cases after tumor shrinkage achieved by primary chemotherapy. Wide resections are generally considered adequate to obtain local control, differently from adult STS that in general should require compartmental resection. In case of primary marginal resection, primary re-operation (prior to any other treatment) is recommended when feasible, hoping to achieve clear margins and proving the absence of microscopic residue in order to avoid radiotherapy [36].
Recently, a possible role for debulking procedure has been suggested for huge retroperitoneal and pelvic RMS [37]. This issue remains, however, controversial, particularly when these surgeries necessitate mutilation. What to do in cases of masses that remain after finishing treatment is debatable; biopsy may cause difficulty in interpreting results, in particular when mature rhabdomyoblasts are detected [38, 39].
Finally, surgery of positive regional lymph nodes is generally considered a diagnostic procedure: any involved lymph nodes warrant radiotherapy, so initial radical lymphadenectomy (which carries a high risk of morbidity) is unnecessary.
Special Situations
Orbital RMS
Orbital RMS carries an excellent outcome, probably reflecting favorable biological behavior combined with early diagnosis because of the location. Embryonal histology is present in approximately 90 % of these cases [40]. The surgical approach to these patients is limited to initial biopsy. Complete resection or exenteration is limited to patients who have local failure following radiotherapy. In a review of pooled data from different studies conducted in Europe and North America, the 10-year EFS and OS were 77 % and 87 %, respectively. Eighty percent of patients received radiotherapy as part of primary therapy. Although more patients who did not receive radiotherapy had local relapse, OS was excellent regardless of the use of radiotherapy, since many relapsing cases were salvaged with radiation and more systemic chemotherapy [40]. The current recommendation in COG protocols is to treat these patients with radiotherapy (reduced dose of 45 Gy), while in Europe the use of radiotherapy is left to the discretion of the treating institution (recommended in Italy and not in France).
Parameningeal RMS
This group of tumors arising in middle ear/mastoid, nasopharynx/nasal cavity, parapharyngeal space, paranasal sinuses, pterygopalatine, and infratemporal fossa region represents a special challenge (Fig. 21.2). Complete resection is rarely feasible even after chemotherapy (difficult accessibility parameningeal sites, risk of mutilation) and radiation therapy must imply high-doses and wide fields, with risk of serious sequelae, in particular in young children. Initial attempts to improve survival by cranial radiotherapy or intrathecal chemotherapy were of no proven value [41]. The cornerstone of local control remains well-planned conformal radiotherapy, though recently, IMRT and proton radiotherapy emerged as viable choices for better delivery of radiation without compromising outcome [42, 43]. COG recommend early radiotherapy (<2 weeks after initiating systemic treatment) in patients with meningeal impingement (defined as cranial nerve palsy, cranial base bone erosion with or without intracranial extension). Despite increased long-term morbidity in infants and toddlers, radiotherapy remains necessary to achieve local control [44].
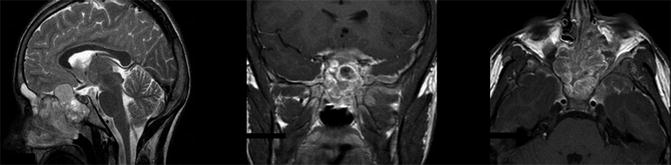

Fig. 21.2
Magnetic resonance imaging of a 13-year-old patient with a huge alveolar rhabdomyosarcoma arising from nasopharynx/nasal cavity and sphenoidal region, with cranial base bone erosion, intracranial extension, and meningeal diffusion
Paratesticular RMS
Paratesticular RMS generally have a good prognosis, in the range of 90 % survival [7]. This is probably due to the peculiar superficial location that allows early diagnosis and complete surgery in most cases, but perhaps also due to a general favorable biology (the adverse prognostic role of alveolar subtype would be counterbalanced by the favorable site, for example) [45].
Paratesticular RMS should be resected, associated to orchidectomy, via an inguinal excision. European groups do not require surgical evaluation of retroperitoneal lymph nodes as routine staging procedure in paratesticular RMS [7], while biopsy is recommended in COG study in patients over 10 years old, considered at major risk to nodal involvement.
Bladder/Prostate RMS
These tumors are typically seen in young patients. Bladder tumors tend to grow intraluminally (Fig. 21.3), in or near the trigon. Prostate tumors usually produce large pelvic masses. Historically, the best approach for local control was believed to be complete excision with margin clearance, which was often pelvic exenteration or aggressive resection with serious complications. However, whether this approach should be always required remains debatable [46], and in some cases a less aggressive surgery with organ preservation may be considered a better option. Brachytherapy may be indicated, providing adequate local control with least morbidity [47].
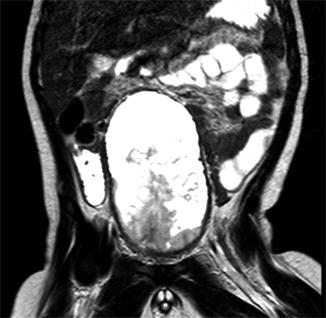

Fig. 21.3
Magnetic resonance imaging of a 2-year-old patient with an embryonal rhabdomyosarcoma of the bladder: the tumor grew intraluminally to completely fill the organ
Young Patients
Patients less than 1 year old at diagnosis continue to have worse prognosis in comparison to older children (1–10 years old). Whether this is the result of different biology of the tumor or treatment modifications that are practiced (e.g., reduction of chemotherapy does, omission of radiotherapy) is not clear yet [48, 49]. Practical general roles in the management of infants with RMS may be the following:
Careful dosing of chemotherapeutic agents to avoid hepatotoxicity (e.g., VOD).
In case of initial reduction of chemotherapy doses, these should be increased in subsequent cycles if therapy has been well tolerated.
Maximum surgical resection to compensate for the high complication related to radiotherapy: amputation may be considered for extremity unresectable tumors since the long-term functional outcome of an irradiated limb may be much worse than amputation.
Careful planning of radiotherapy; although decreasing treatment volume is not well established based on available data, the decision to decrease volume should lean toward reducing toxicity in this age group.
When decisions are made to decrease treatment, survival should remain as the main target of treatment. This was shown by the International Society of Pediatric Oncology—Malignant Mesenchymal Tumour Committee (SIOP-MMT) approach to young children with parameningeal tumors, where treatment reduction resulted in unacceptable low survival [44].
Relapsed RMS
Relapsing patients remain one of the greatest challenges in the management of RMS. Approximately, one third of these patients can be expected to be alive at 3 years. Actual long-term cure remains to be possible in a minority of patients, in particular, those who relapse locally and did not receive radiotherapy as part of their initial therapy fare better [50]. Aggressive surgery and second-line drugs should be considered; however, it may be said that in countries with limited resources, treating patients with recurrent metastatic disease may be generally of little value, unless it is directed to proper palliative care.
Challenges in Developing Countries
It is generally considered that the therapeutic standards achieved in developed countries in RMS are unlikely to be reproduced in low-income countries, due to the differences in health infrastructures and training, the limited availability of some active drugs and supportive care to face life-threatening toxicities of modern chemotherapy, and the poor treatment compliance by patients. Nevertheless, the quality of care in developing countries is rapidly increasing.
A limited number of RMS series in developing countries has been published [25, 51–55] (Table 21.4).
Table 21.4
Published rhabdomyosarcoma series by developing countries
Study | Country | Number of pts | Results | Comments |
|---|---|---|---|---|
Al-Jumaily et al. [25] | Jordan | 45 pts | 4-year PFS = 61 % | Improved outcome in more recent years with multidisciplinary care and protocol adjustments |
4-year OS = 72 % | ||||
Badr et al. [54] | East Egypt | 41 pts | FFS = 68 % | Metastatic disease = 39 % |
OS = 57 % | ||||
Wood et al. [51] | South Africa | 49 pts with genitourinary RMS | OS = 65 % | More advanced tumors compared to the literature |
Better in pts treated after 1992 (80 %) | ||||
Friedrich et al. [53] | Central America | 240 RMS among 785 pts with sarcoma | 4-year EFS = 33 % | High rate of metastatic disease at diagnosis; treatment abandonment = 25 % |
4-year OS = 44 % | ||||
Hessissen L et al. [52] | Morocco | 100 pts | 10-year EFS = 39 % | Treatment abandonment = 37 % |
10-year OS = 70 % | ||||
Antillon F et al. [55] | Guatemala | 47 pts | 3-year EFS = 26 % | Difficulties in local control; abandonment = 30 % |
3-year OS = 43 % | ||||
Shouman et al. [56] | Egypt | 190 pts | 5-year FFS = 40 % | No standardized protocols |
5-year OS = 50 % |
Multiple factors seem to have a role in affecting RMS patient outcomes in developing countries. In addition to the general socio-economic factors that adversely affect the care of children with cancer in countries with limited resources, negative factors that may be more specific for RMS include:
1.
The problem of delayed diagnosis and advanced stage of disease at diagnosis, related to the difficulty in referral to specialized centers and the poor access to healthcare in general; delay in diagnosis in RMS has been demonstrated to be a significant prognostic factor [57].
2.
The high percentage of abandonment of treatment prior to its completion (particularly when transportation is a challenge), probably due to refusal to radical local control and the need for long treatment plan.
3.




Intensive chemotherapy toxicity; patients with malnutrition are at particular risk, and the lack of supportive care including the lack of well-equipped intensive care units and the cost of growth factors make it difficult to provide treatment for high-risk patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree