Tumour stage/histology
Radiotherapy (RT) dose and fields
1/FH and/2 FH
No RT
3/FH, 1–3/FA 1–2/DA 1–3/CCSK
10.8 Gy flank RT
Ascites (cytology +ve)
10.50 Gy whole abdomen RT (WART)
Pre-op tumour rupture
If residual tumour, boost with further 10.50 Gy
Diffuse surgical spillage
Peritoneal seeding
3/DA 1–3/RTK
19.8 Gy (infant 10.8 Gy)
4 (Lung, FH)
12 Gy whole lung irradiation (WLI) if no complete remission (CR) at week of 6 triple chemo
4 (Lung, UH)
12 Gy WLI regardless of (CR) or not
4 (Brain)
21.6 Gy whole brain + 10.8 Gy boost <16-year-old
4 (Bone)
25.2 Gy (tumour + 3 cm)
4 (Liver)
19.8 Gy to focal metastases if unresectable
Unresected LN
19.8 Gy
Resected LN
10.8 Gy
Abdominal relapse
12–18 Gy (>12 m)
For SIOP, the decision for RT is also based on post-operative risk assessment, but this is post–chemotherapy:
Risk assessment
Low risk (LR): Mesoblastic nephroma, cystic partially differentiated nephroblastoma and completely necrotic nephroblastoma.
Intermediate risk (IR): Epithelial, stromal, mixed, regressive, or focal anaplasia type nephroblastoma.
High risk (HR): Blastemal, diffuse anaplasia (DA), clear cell sarcoma of the kidney (CCSK), rhabdoid tumour (Table 14.2).
Tumour stage/histology
Radiotherapy dose and fields
2 HR except blastemal
Flank 14.1 Gy
3, IR
Flank 14.4 Gy ± boost 10.8 Gy
2, 3, HR
Flank 25.2 Gy/14#
3 with diffuse spillage
WART 14.1 Gy ± boost for gross disease up to 21 Gy
Max 12 Gy for <1 year
Peritoneal metastases
WART 14.4 Gy ± boost
4 (Lung) persistent at 9 weeks, any histology
WLI 15 Gy/10#
4, Any HR tumour regardless of whether lung metastases present
WLI 15 Gy/10#
New lung metastases
WLI 15 Gy/10#
4, Brain
25.5 Gy ± boost of 4.5 Gy
4, Liver
20.05 Gy
Timing and Technique of Radiotherapy
For non-metastatic Wilms tumour, tumour bed RT, when required, should be commenced within 10 days of surgery.
Flank RT
Volumes
These are the same for COG and SIOP. They may be defined using simulator or image intensifier; however, CT-based planning allows superior definition of volumetric doses to organs at risk.
Tumour bed (TB) = outline of kidney + tumour as defined from pre–operative imaging
Clinical target volume (CTV) = TB + 1–2 cm margin taking into account:
Pretreatment tumour size
Surgical findings and clips
Pathology report
Planning target volume (PTV) = CTV + margin for movement and set-up error (about 1 cm) as well as:
Must include full width and length of vertebral bodies included in the radiation field (in order to prevent growth asymmetry later in life).
Must include the lateral abdominal wall.
Whole Abdomen Radiotherapy (WART)
Volumes
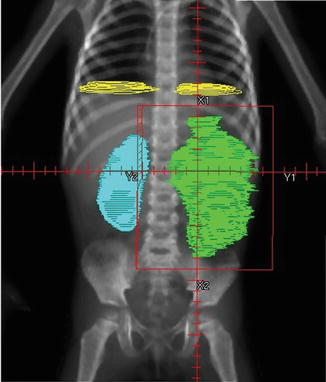
The entire abdominal contents with all peritoneal surfaces are included. Care must be taken to include the entire dome of the diaphragm, and extend down to the peritoneal reflection at the obturator foramina. The heads of femur are blocked. Anterior and posterior fields are used. Note that a margin of 2 cm is required if Cobalt-60 is used (Fig. 14.2).
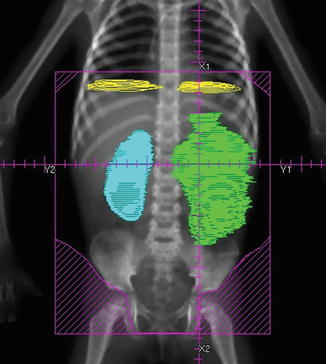

Fig. 14.2
Whole abdomen radiotherapy (WART) for diffuse spillage of a left-sided Wilms tumour. From Halperin et al. [8]
Whole Lung Irradiation (WLI)
This includes both entire lung fields and is treated with anterior and posterior fields. Care must be taken to extend fields to adequately cover both lung apices and inferior recesses. Posterior lung recesses are best seen on a lateral simulation view. If Cobalt-60 radiation is used, a margin of 2 cm on the target is required (Fig. 14.3).
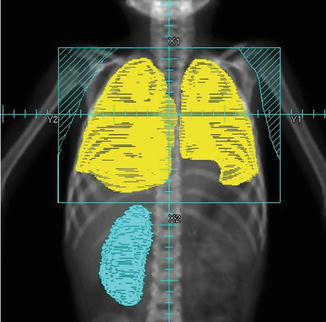

Fig. 14.3
Field for whole lung irradiation (WLI). Lung volumes indicated in yellow. Note that inferior recesses are best seen on a lateral view. From Halperin et al. [8]
If both lung and flank RT are required, these should be simulated and treated simultaneously as a single field. If whole lung and whole abdomen RT are required, these can be treated simultaneously, but in a child with any co-existing problem such as TB or malnutrition, it is advisable to plan the fields simultaneously but treat them sequentially with a 2 week gap between courses. Ideally, the lower half of the field is blocked for lung RT and the upper for the abdomen RT. However, on Cobalt machines, this means very large and heavy lead blocks which are difficult for radiographers to lift. It may therefore be necessary to use two separate fields which require matching. Care must be taken not to overlap fields over the liver.
Boost Volumes
For boost volumes, or treatment volumes requiring higher doses, it is always preferable to use 3-D CT-based planning in order to adequately protect surrounding organs at risk, e.g. single residual kidney or lung. This allows the use of oblique treatment fields, if necessary.
If CT-planning is not available, then simulation or intensifier-based planning is used. This means parallel-opposed fields. Careful plotting of organs at risk is necessary using CT pictures and/or IVP.
Organs at Risk
There is very little literature available on tolerance doses of RT in children. Table 14.3 incorporates information from COG protocols.
Table 14.3
Paediatric radiotherapy tolerance doses (based on COG protocols)
Organ | Dose (cGy) |
|---|---|
Single organs | |
Bladder | 4,500 |
Heart | 3,000 |
Liver-whole | 2,340 |
Liver-partial (50 %) | 3,060 |
Spinal cord | 4,500 |
Rectum | 4,500 |
Peritoneum (whole abdomen pelvis) | 2,400 |
Small bowel | 4,500 |
Paired organs | |
Kidney-whole | 1,440 |
Kidney-partial (50 %) | 1,980 |
Lung—whole | 1,200 |
Lung (when PTV >1/2 bilateral lung volume) | 1,500 |
Lung (when PTV <1/2 bilateral lung volume) | 1,800 |
When the whole abdomen or liver dose exceeds 1,440 cGy, the renal dose should be limited to 1,440 cGy by using appropriate renal shielding.
Several techniques may be used for renal shielding when the whole abdomen irradiation (WAI) dose is >1,440 cGy. The use of posterior partial transmission kidney blocks for the entire course of treatment is recommended.
The thickness of the block will be determined by the treatment plan. The dimensions of the block should be 0.5 cm wider than the projection of the kidney on a PA digitally reconstructed radiograph.
RT for Medulloblastoma
A prerequisite for radical treatment of medulloblastoma is the availability of a fairly sophisticated health system, including all of the items listed below:
Adequate imaging (at least a CT scan) for diagnosis and planning, as well as portal field verification for quality assurance.
Adequate surgery (trained surgeons, anaesthetist, and ICU services).
Pathology services.
Ability to plan craniospinal RT and to treat at least four fractions per week.
Availability of mould room services to manufacture blocks and/or compensators, as well as immobilisation devices adequate for reproducible and accurate field matching.
Craniospinal irradiation (CSI) is one of the most difficult techniques for any radiotherapy planning department or treatment floor. It requires advanced planning techniques in order to limit toxicity and these have evolved considerably over the past 15 years. It also requires reproducibility, and accurate set-up, in order to match fields [10].
Although CSI can be planned using an image intensifier or simulator together with a 2D planning system alone, this is extremely challenging and is associated with increased side effects. CT-based three-dimensional (3D) planning is easier and less time-consuming; however, it requires specialised equipment and software which is expensive and therefore is not available in many LICs. Similarly, although it is possible to administer CSI from a Cobalt-60 machine as opposed to a linear accelerator (Linac), it is much more difficult to achieve an acceptable plan and toxicity is greater.
General principles of treatment:
Patients usually present acutely to neurosurgery with hydrocephalus. Initial diagnosis is made on imaging and then confirmed by histology after resection. Ideally spinal staging using MRI is done preoperatively, but in LICs and MICs this is frequently not feasible. Staging CSF sampling is done by lumbar CSF tap at least 2 weeks post-surgery. Immediate cytospin and microscopy of CSF may show tumour cells.
Staging is done post-surgery (Table 14.4) [11]
High risk
<3 years
Leptomeningeal disease
Residual tumour >1.5 cc
Average risk
≥3 years
M0 on staging investigations
Gross total resection
All children with medulloblastoma require CSI if cure is the aim.
It is arguable that radical surgery should not be attempted in situations where CSI is not available, as no cure is possible. In this event, hydrocephalus should be shunted to relieve symptoms, and the child palliated.
Children under 3 years of age require chemotherapy and surveillance until they are over 3 years of age, when they should receive CSI. They are classified as high risk. In many LICs and MICs, this may not be feasible. In such cases, these children should be palliated.
Dose of CSI
1.
The dose used for CSI depends on several factors:
(a)
Ability to accurately stratify patients into average risk and high risk groups. (This requires MRI spinal imaging and/or CSF cytology.)
(b)
Availability of chemotherapy.
(c)
The ability of the family to bring the child for 6 cycles of adjuvant chemotherapy after CSI.
If the answer to any of the above is “no”, then full dose CSI is given regardless of risk assessment. For patients who receive full dose CSI for logistical/practical reasons, no concurrent chemotherapy is administered (Table 14.5).
Table 14.5
Dose of craniospinal irradiation (CSI) in medulloblastoma
Craniospinal axis | Posterior fossa boost | |
|---|---|---|
Full dose CSI (high risk) | 36 Gy/20# (5× per week) | 19.8 Gy/11# (5× per week) |
Reduced dose CSI (average risk)—given with chemotherapy | 23.4 Gy/13# (5× per week) | 30.6 Gy/17# (5× per week) |
For patients assessed as “average risk”, and who are able to get chemotherapy, reduced dose CSI is given, together with weekly Vincristine during CSI and 6 cycles of adjuvant chemotherapy thereafter [12].
For patients who are assessed as “high risk”, they should receive full-dose craniospinal RT as well as 6 cycles of adjuvant chemotherapy.
Timing of RT
RT treatment should be commenced within 40 days of surgery, and preferably within 28 days of surgery. Delays may lead to a worse prognosis.
If delays are expected due to the patient’s condition or planning delays, 1–2 cycles of chemotherapy can be given prior to starting radiotherapy.
During RT
All patients require weekly full blood count (FBC) testing as a significant amount of bone marrow is in the field and pancytopenia may result. Delays are reserved for the unusual cases of extreme cytopenias and are avoided if at all possible.
Many patients require anti-emetics during the CSI as a significant amount of small bowel and stomach is in the field and nausea/anorexia is a common side-effect. Treatment checks are done weekly and should include weekly FBC to look for bone marrow suppression due to treatment.
Skin reaction around the pinna and neck is treated with 1 % hydrocortisone cream.
Food and fluid intake and a weekly weight should be monitored in children, as many don’t eat due to anorexia, nausea, or prolonged sedation.
All patients require long-term follow-up for late toxicity of treatment including:
Neuro-cognitive problems
Psychosocial problems
Pituitary and thyroid hypofunction
Osteoporosis
Cardiovascular problems
Second malignancy
Technique for CSI
Patients may be treated prone or supine for CSI. Supine treatment has many advantages [13], but requires that the beams are treated through the treatment couch and head rest. The treatment planning system must therefore have the ability to account for transmission through the couch or immobilisation devices that are in the posterior fields. This is not possible for older couches which have metal inserts. In this instance, prone treatment is mandatory and a custom-made body foam/shell and prone head cast are required for patient positioning and immobilisation. For supine treatment, an orfit cast can be used and is extended to below the shoulders.
CT-planning is preferable, although several techniques for simulation and 2D planning have been widely used [10].
The patient MUST be positioned straight with the help of lasers.
The head should be placed in a slightly extended position and such that the cervical spine is as flattened as possible.
Beam Arrangement
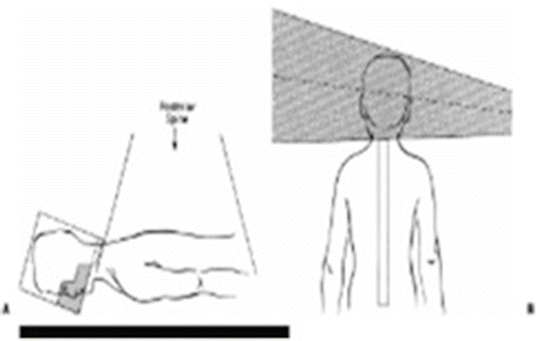
CSI is composed of two lateral head fields and a contiguous spinal posterior spinal field (Figs. 14.4 and 14.5).
The lateral head fields have a collimator rotation to match the superior beam edge of the spinal field. The head fields must be positioned to clear the shoulders.
Face shielding is accomplished by custom-made lead blocks if MLC (multi-leaf collimator) is not available.
○ The superior edge of the block should be situated 0.5 cm below the cribriform plate. It is important not to underdose the cribriform plate as this is bathed in CSF and is a site for recurrence in medulloblastoma.
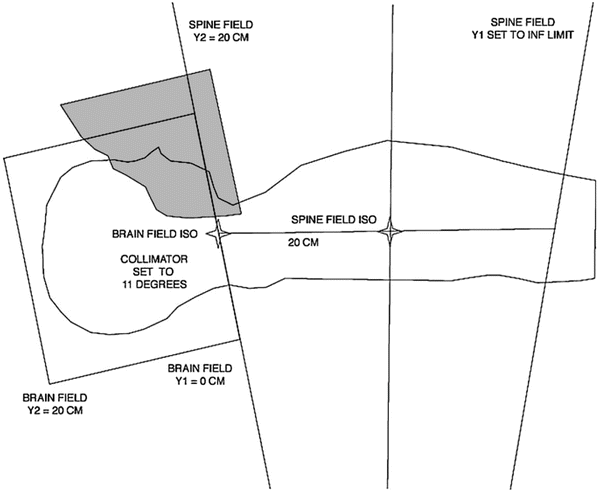
○ The block should be placed 1 cm anteriorly to the middle cranial fossa, 1 cm inferiorly to the base of skull and extend posteriorly down the posterior pharyngeal wall. These bony structures should be identified when placing the block (Fig. 14.6).
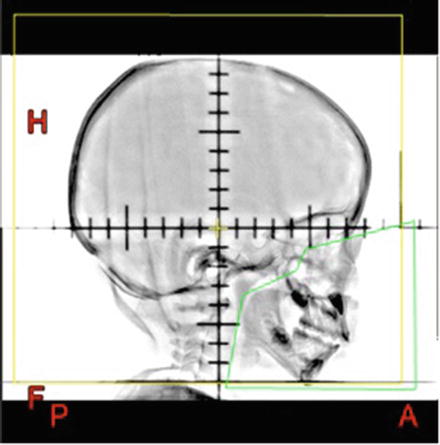
Fig. 14.6
Customised face block for lateral head fields
The spinal fields, if not 3D planned, are simulated with a daily small couch rotation, alternately clockwise and anticlockwise, to match the beam divergence of the lateral head fields.
The junction between head and spine fields should be “feathered” by moving the junction by 1 cm at least twice during the course of CSI in order to minimise cold or hot spots caused by the junction/set-up error, etc.
The boost field can be treated as two parallel opposed lateral fields, with the posterior fossa field defined on a lateral skull X-ray, OR by a more conformal 3D planned volume (tumour bed + margin of 2 cm), defined on CT and planned with 3–5 fields [14].
Ideally all fields should be summed and a total Gy plan produced.
Treatment
It is critical to check the position of the fields prior to treatment. This is done on the simulator prior to starting treatment, or by portal imaging on the machine.
The position of the axes as well as the position of the block relative to bony structures is checked. This is accomplished in the head fields, by a double exposure with and without the block, in order to visualise the bony anatomy behind the block.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree