Antineoplastics
Section
Antimicrobials
Section
Supportive care agents
Section
Asparaginase
8.2
Acyclovir
6.4.1
Allopurinol
8.2
Bleomycin
8.2
Amikacin
6.2.4
Amitriptyline
24.2.1
Carboplatin
8.2
Amphotericin B
6.3
Codeine
2.2
Cisplatin
8.2
Cefotaxime
6.2.1
Diazepam
5
Cyclophosphamide
8.2
Ceftazidime
6.2.1
Docusate
8.4
Cytarabine
8.2
Ceftriaxone
6.2.1
Folinic acid
8.2
Dacarbazine
8.2
Clindamycin
6.2.2
Heparin
10.2
Dactinomycin
8.2
Fluconazole
6.3
Lactulose
8.4
Daunarubicin
8.2
Gentamycin
6.2.2
MESNA
8.2
Dexamethasone
8.3
Meropenem
6.2.1
Midazolam
8.4
Doxorubicin
8.2
Metronidazole
6.2.2
Morphine
8.4
Etoposide
8.2
Septra
6.2.2
Ondansetron
8.4
Hydrocortisone
8.3
Vancomycin
6.2.1
Senna
8.4
Hydroxyurea
8.2
Ifosfamide
8.2
Mercaptopurine
8.2
Methotrexate
8.2
Methylprednisolone
8.3
Prednisolone
8.3
Thioguanine
8.2
Vinblastine
8.2
Vincristine
8.2
In order to promote the development of drugs with pediatric indications and to provide more pediatric dosing, labeling, and safety data; regulators such as the United States Food and Drug administration (FDA) and the European Medicines Agency (EMA) have enacted legislation to mandate basic pediatric data for new drug submissions and financial incentives for drugs already licensed or “off-patent” [30–32]. The impact of this legislation is currently debatable, since all of the additional data provided by manufacturers have been for drugs that are still “on-patent”; and in Europe, of 29 new anticancer drugs licensed by 2009 only 6 had a pediatric indication. Furthermore, the new pediatric information (dosing, safety, labeling) generated by manufacturers as a result of these initiatives is not being filed in other jurisdictions because there is no financial gain to be had from doing so [33–35].
Infrastructure and Logistical Issues
Regulatory agencies in high income countries such as the FDA, the Health Protection Branch (Canada), and the EMA, undertake reviews of data submitted by drug manufacturers with respect to efficacy, safety, and quality, as well as performing cost-effectiveness analyses before granting a notice of compliance (NOC) with their respective regulations and issuing a license to manufacture and/or sell the product within the respective country/jurisdiction. Developing countries may not have such agencies in place and, if they do, likely these will not have the capacity or expertise to adjudicate such submissions properly. These countries rely on international agencies such as the WHO for guidance and programs that can assist with drug evaluations. One of the fundamental guiding documents is the WHO Model List of Essential Medicines (adult and pediatric) [27, 28] which health ministries in LMICs can access and tailor to the needs of their populations. This is done best in conjunction with published evidence-based treatment guidelines that now exist for many medical conditions. A proposed framework is shown in Fig. 10.1 [36].
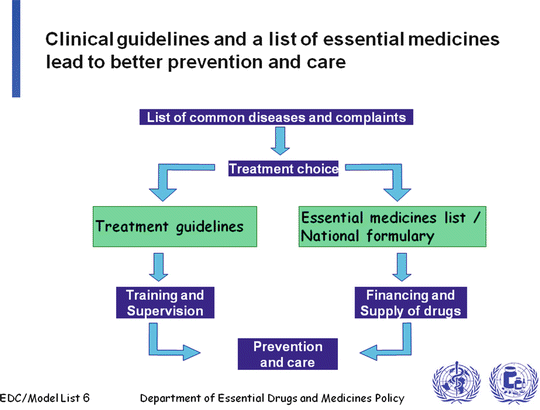

Fig. 10.1
Potential framework for policy development for medications in low and middle income countries
The quality of drugs available in developing countries is also of increasing concern [29, 37, 38]; since many chemotherapeutic agents are clear colorless solutions and, beyond emesis with certain agents (e.g., cis-platinum), have no immediate clinical/laboratory value correlates for “fraud,” making them easy prey for counterfeiters, with estimates that up to 30 % of drugs in certain settings may be counterfeit or substandard [39]. Using the adage of “you get what you pay for” attempts have been made to use “low-price” together with “brand vs. generic” and “appearance” of the selling pharmacy as indicators of poor quality drug product in LMICs; with low price for a “brand” drug being somewhat useful, but none of these factors is highly helpful [40]. Due to the high cost of imported brand name drugs, some governments in LMICs have fostered the development of generic pharmaceutical industry capacity within their own borders (e.g., India), or import generic versions of drugs preferentially. This is sound policy and can result in significant cost savings [41]; however, no strategy is perfect and tragedies have occurred [42, 43]. To address the issue of access to high quality pharmaceutical products, the WHO developed a “Prequalification Program” in 2004 for manufacturers of drugs. The program encompasses all major aspects of drug manufacture and quality assurance and provides for inspections as well as employing quality control laboratories. Drugs that pass quality control are published on the WHO prequalification website for reference by governments and NGOs purchasing drugs for LMICs [44].
Additional factors that impact on drug availability and access in developing countries are infrastructure issues. Due to lack of local production, many drugs in the developing world have to be imported. Once imported, distribution of such drugs to health care providers in hospitals, clinics, or other medical facilities may be problematic. Roads may be in poor states of repair; some drugs require storage under controlled temperature/humidity conditions or refrigeration, which may be problematic during transport; or, at the destination, if there is no refrigerator or provision of consistent electrical supply.
The social and economic conditions in developing countries often make them fertile breeding grounds for corruption. Life saving/prolonging drugs such as antineoplastics and antiretrovirals are often stolen and resold on the black market. Narcotic analgesics have high resale value and, coupled with concerns about addiction, many governments in LMICs have adopted overly restrictive drug control policies at the national level [45, 46]. This is particularly tragic as it results in pain being the most undertreated symptom in patients in the developing world; and, for the child (or adult) with cancer whose chances of cure are low, effective palliation is virtually impossible.
While the lack of child-friendly formulations is true for most drugs, it is particularly true for antineoplastics. Injectable drugs are available almost universally in sizes geared towards adult patients; this is problematic globally and not unique to LMICs. However, given the cost of these agents, and potential lack of proper storage facilities (e.g., refrigeration), enormous waste occurs if a second or third child cannot benefit from a dose of drug that has been reconstituted. In some cases, the provision of cancer care (adult and pediatric) is undertaken at a single center; this may be a preferable scenario in LMICs as it provides the opportunity to realize cost savings through minimization of waste. Indeed, in some settings, doses of chemotherapy for adults are rounded to the nearest vial size for this reason. Oral agents are equally problematic in the lack of appropriate dosage forms for children, necessitating the handling of antineoplastics to split tablets or open capsules by health care personnel or parents/caregivers without the availability of proper personal protective equipment.
The Role of Pharmacists in Cancer Care
Pharmacists are society’s experts on medicines. They work at the interface between the vast global drug development and marketing apparatus, and the patients who are prescribed these drugs or consume “over the counter” preparations. Their primary role in today’s health care environment is to promote and support the safe, effective, and rational use of medications. Globally however, there remains significant variability in how pharmacists actually practice. In many resource-rich countries, pharmacy practice has and continues to evolve with pharmacists now being more engaged in direct patient care, working together with physicians and nurses to ensure the best outcomes for patients in both hospitals and the community. Occurring in parallel with this evolution has been the development of credentialing bodies such as the Board of Pharmacy Specialties [47] in the United States which establishes criteria for specialty practice areas and standards for credentialing of pharmacy specialists; with oncology pharmacy practice being a specialty. Indeed, some of the fundamental dispensing functions performed by pharmacists are now being devolved to properly trained pharmacy technicians, freeing up pharmacists’ time to devote to patients. Given the complexity of caring for patients with cancer, requiring multidisciplinary teams [48], the costs of antineoplastic drugs, the potential for severe drug toxicity in the event of a medication error, and requirements for the safe preparation, administration, and disposal of cytotoxics, pharmacists are critical members to include in the health care team. This is no less true in LMICs than it is in resource-rich countries; although it must be acknowledged that in some settings, access to a pharmacist is not possible, and the role of chemotherapy preparation and administration falls to a nurse. It can be argued that having pharmacists involved in the care of patients with cancer in LMICs is as (or more) vital than in a high income country. Given the risks for inadvertent exposure to antineoplastic drugs by health care personnel, establishing a dedicated oncology pharmacy and having a trained pharmacist (or pharmacy technician, depending on the setting) to prepare chemotherapy (ideally utilizing appropriate personal protective equipment and a biological safety cabinet or laminar flow hood) and properly dispose of used administration sets afterwards is of prime importance. Additional benefits of having a dedicated oncology pharmacist and pharmacy service relate to establishment of standard operating procedures and documentation of workload leading to better inventory control. By establishing and projecting drug needs, it may be possible to purchase drugs in bulk and realize additional cost savings. Additionally, having a secure central storage facility may prevent loss due to theft. The key elements in establishing an effective oncology pharmacy in an LMIC (Kenya) are listed in Table 10.2 and a core curriculum for pharmacists’ training (Table 10.3) have been described recently by Strother et al. [49]. While there are no distinct standards of practice for oncology pharmacy in LMICs, the International Society of Oncology Pharmacy Practitioners (ISOPP) has developed and published Standards of Oncology Pharmacy Practice that take into account realities from both resource-rich and resource-poor settings. These standards address key issues for pharmacists and nursing personnel preparing and administering chemotherapy with respect to sterile preparation, personal protective equipment, waste and spill management as well as management of drugs and checking procedures to prevent medication errors [50]. A second edition of the standards is to be published in 2013. Beyond their primary functions described above, pharmacists in LMICs can participate in additional initiatives such as establishing pharmacovigilance programs [51] and contribute to patient care through the use of standardized assessment tools for toxicity and adherence, and in patient/parent education [52–55].
Table 10.2




Recommendations for development of an oncology pharmacy in a resource-limited setting
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree