There is a tremendous need for the integration of palliative care into paediatric oncology services in developing countries. This is in large part due to the relatively high mortality of children with cancer in these countries, and the frequent association of cancer and its treatment with pain and other symptoms [9]. While palliative care teams have expertise in symptom management, communication and end-of-life care, such services often are not available in developing countries, and it therefore falls to the paediatric oncology team to provide these services.
Communication and Palliative Care
Communication with seriously ill children and their families is a central part of providing good palliative care. While palliative care physicians have expertise in this realm, it is incumbent upon paediatric oncologists to develop some level of comfort and expertise as well, because they are usually the physicians directing care of the child with cancer, and families look to them for information and guidance. Communication can be a daunting challenge, because paediatric oncologists must often communicate difficult news (new diagnosis of cancer, recurrence, limits of treatment options), and must discuss goals and limits of care. It is important to reach out to the parents and child, and explore their hopes, concerns and goals of ongoing care. Information should be shared adjusting the style of communication to the family’s level of knowledge and prior understanding/misunderstandings. Families rarely hear or understand all that is said to them, even after much repetition. They often find it difficult to formulate questions in context of an emotional interview carried out in a hospital [10]. Perhaps the two most common and challenging areas of communication are delivering bad news and discussing goals and limits of care (e.g. resuscitation), each of which are discussed below.
Sharing bad news. Paediatric oncologists are frequently required to share difficult news with children and families, whether it be the initial diagnosis of cancer, recurrence of disease or that the child’s disease is no longer curable. Regardless, communication at this point needs to be handled sensitively [4]. The news must be given clearly and should respect the family’s coping strategies. Many physicians follow a six-step protocol, described by Buckman et al. [11]: (1) Prepare. Find a quiet place, turn beepers off or on silent, making sure that one has the time needed to devote to a family, and that the family has the people they want/need present at the meeting. (2) Find out what the patient/family knows. Ask them what they already know, understand and perceive. This gives insight into their factual knowledge as well as their understanding of what is happening. (3) Find out what the patient/family wants to know. Allowing families to assist in “setting the agenda” can transform the “bad news” discussion into an opportunity to reassure and support. (4) Share the information. Information should be given to families at a level, pace and amount they can assimilate easily and accurately. The message they receive is not always the message that is given. For example, families may know that multiple metastases have developed, but may not know that means cure is impossible [12]. Check in with them along the way, saying things such as “Does this make sense so far?” or “Do you want to ask me anything about what I have just said?” It is not uncommon for families to misinterpret or not remember what is said. It is not because the oncologist failed to speak clearly, but because they find it hard to take everything in. (5) Respond to emotions. Perhaps the most important aspect of a “bad news” conversation, this is a time to listen, support and allow periods of silence for parents who are too emotionally overwhelmed to process what they have just been told. (6) Provide a follow-up plan. Families may leave a difficult conversation like this stunned and lost. It is vital to have a specific follow-up plan so they know they are not being abandoned and so they can ask questions they might not have had the presence of mind to ask at the initial meeting. Table 11.1 outlines a few key points in “How to deliver bad news”, similar to the approach taken by Buckman et al. [11]. Regardless of the precise protocol followed, the key to effective communication is to be sensitive, listen, have empathy for the child and family’s concerns, respect their cultural and spiritual beliefs and do not be judgemental [2].
Set the scene | • Find a private and quiet place |
• Invite relevant others—child, social worker, ward staff, etc. | |
• Ensure eye-contact on the same level (ideally all sit) | |
• Speak in an unhurried way | |
• Never look at clock or watch | |
• No interruptions (give bleep to someone else) | |
Alignment (let the family talk) | • What does the family already know? |
• What does the family understand? | |
• What are they expecting from you in this interview? | |
Exchanging information | • Acknowledge what is already known and understood |
• Use it as basis for giving new information—use the family’s vocabulary | |
• Prioritize: what are most important and/or immediate concerns? | |
• Repeat and summarize as often as necessary | |
Checking back | • What has the family understood? |
• Did they receive the message you intended to give? | |
• Have you answered what they needed to hear? | |
• Go back to step 3 as often as necessary | |
Closing | • Reassure the family that they are not expected to remember everything |
• Invite further questions (go back to step 3 if necessary) | |
• “Permit” further questions in the future—of you, ward staff, etc. | |
• Make arrangements to meet again | |
• Summarize once again |
Talking to children. Many parents and professionals find it difficult to involve children in discussions about a diagnosis of cancer, particularly if their prognosis is poor. It is the instinct of parents and professionals alike to protect children from “badness”. However, there is a wealth of evidence that suggests most children know when they are dying, feel isolated when such information is withheld and fare better when they are told what is happening [13]. Much of this evidence comes from the United States and western Europe and may not hold true for other parts of the world where cultural norms regarding sharing information may differ. As for who and how to talk to children, it need not be the physician, and in fact, for older children it is best to explore their hopes and fears with whomever they feel comfortable and have a connection. That might be their parents, a chaplain, a nurse, psychologist or social worker [10]. For younger children, child life therapists are particularly skilful in helping children express themselves through play therapy.
Siblings. Just as it is difficult to involve children with cancer in discussions of poor prognosis, so, too, is it difficult to have these discussions with their siblings. There are data which suggest that siblings, too, fare better when they are fully informed about their brother’s or sister’s illness. SIOP has issued suggested guidelines for helping siblings cope with their siblings’ illness and/or impending death [14].
Challenges regarding communication in developing countries. The approach to sharing bad news directly with patients, be they adults, parents or children, is highly variable from country to country and culture to culture (even within the same country) [15]. In the United States and western Europe only a minority of physicians disclosed a diagnosis of cancer or a poor prognosis as recently as the 1950s and 1960s. But since that time, there has been a trend toward full disclosure to the point that now full and open communication with patients (even children) is standard practice. However, this approach does not hold true in many parts of the world where full disclosure of diagnosis and prognosis is often withheld from the patient (although shared with the family) [15, 16]. With such cultural variability in how difficult news is approached, it is essential for the paediatric oncologist to ask parents how much detail they want about their child’s illness, and how to approach these issues with their ill child and his/her siblings.
Goals of care. When a child with cancer is not doing well, when treatment options are limited or when it appears that the child’s disease is not curable, it is important for the physician to communicate this to the parents and the child (if appropriate). At this point the paediatric oncologist must work with the family to decide what sort of medical interventions make sense to do and which ones do not. For example, will it really help the child with refractory leukaemia to receive one more round of chemotherapy, or will this most likely have significant toxicity and little efficacy. Or does it make sense to give a child artificial nutrition or hydration if it does not enable the child to live longer and does not provide comfort. In a developing country where resources may not be available for curative treatment, these conversations may need to take place early in children’s course, even at presentation.
Do not resuscitate orders. Perhaps one of the most emotionally charged aspects of goals-of-care discussions is cardiopulmonary resuscitation (CPR). For children who have relapsed and who have incurable disease, the benefits of CPR are questionable, and the potential toxicity considerable. Whenever possible, discussion of CPR should be done in advance to allow parents and children to make decisions that would fit with their values and goals of care.
When having such a discussion, it is important to honestly assess the likelihood of benefit versus harm performing CPR. It should be handled as any other medical recommendation: the physician should take responsibility for the process and not put undue responsibility for any decision on the parents. It should be a process of shared decision making with the medical team taking the lead, guiding the family, but also listening to and including them. It should be pointed out to the parents that it would be the uncontrolled cancer causing the death and not their decision not to resuscitate. In addition they should be reminded that “Do not resuscitate” (DNR) status does not mean “do nothing”. It simply means that one does not proceed with this intervention because it is unlikely to work and is likely to cause harm. Even if parents are unable to make a decision, it is helpful for them to have heard that they may have to face such a decision in the future [2, 6].
The appropriateness of DNR discussion may also depend on the resources available in a given country. For example, in some resource-poor countries a child with ALL might have a very poor prognosis. In such a case, DNR might be discussed early in the child’s course. In other countries resources for conducting advanced CPR might not be available so such a discussion is not relevant. In addition, laws may vary from country to country regarding the legal requirement to discuss resuscitation status with families [17].
Location of Care for the Dying Child
Whether a child dies at home or the hospital, early discussion and planning of location of death is highly recommended. When such early discussion takes place, families and children fare better at end of life and children are more likely to die in their preferred location [18].
If families opt for home care, the paediatric oncology team is obligated to ensure that good palliative care is provided in the child’s home and/or community. For many families the paediatric oncologist will still remain their key medical professional. Most of the time the key team members in the community will be the community nurse, general practitioner (GP), paediatrician, etc., with the paediatric oncologist simply providing occasional guidance and support. Some children and families require input from psychologists, chaplains and, if available, pain specialists and child life specialists. Where available an outreach nurse and/or children’s hospice are valuable resources to provide effective palliative care [12].
Although the inpatient paediatric oncology unit may not seem to be the ideal place for end-of-life care, for some families that may be where they are most comfortable. In addition, sometimes hospital admission is unavoidable (due to pain control, the need for transfusions, etc.). In this instance, the goal should be to create a home-like environment. If available a single room should be set aside and allow the family to decorate it with toys and home furnishings, etc. as they see fit. It is important for team members to take time to talk to the family members each day, even though there is no active treatment of the cancer. Generally, this does not take as long as one might expect and a great deal can be said in a 5- or 10-min encounter [12].
Symptom Management
Management of symptoms is a very big part of paediatric palliative care. Children with cancer experience a myriad of symptoms (physical and psychologic) from the tumour and the therapy from the time of diagnosis until the time of cure or death. A good working knowledge of the most common symptoms experienced by children with cancer, how to assess these symptoms and how to treat them is a cornerstone of good paediatric palliative care.
Pain Pain is one of the most common and most debilitating of symptoms. While there are many definitions of pain, perhaps the most compelling one was written by a nurse over 40 years ago: “Pain is whatever the experiencing person says it is, existing whenever the experiencing person says it does” [19]. In the United States, pain is now considered “the 5th vital sign”, and is assessed regularly in US hospitals and clinics along with pulse, blood pressure, respiratory rate and temperature. Optimal pain management begins with a good assessment including biological, psychological, social, cultural and spiritual factors that influence the way a child perceives pain. Such assessment is simple, takes little time and is feasible in resource-limited settings [12].
Pain assessment The purpose of pain assessment is to determine the severity and nature of pain. The assessment should include a detailed pain history (see Table 11.2 which highlights the PQRST mnemonic) and a physical examination. Further investigation of the underlying cause of the pain depends on where a child is in the course of his/her illness. For example, evaluation of abdominal pain in a child who has just started chemotherapy for a newly diagnosed malignancy might be extensive, whereas exhaustive evaluation of the cause of bone pain in a child with end-stage leukaemia is not necessary [12].
Table 11.2
The following aspects of pain should be recorded and is summarized by the useful mnemonic “PQRST” [4]
Precipitating or relieving factors | • Abdominal pain relieved by defecation suggests that constipation is the cause |
• A pathological fracture can be suggested by pain on movement of the affected limb | |
• Also pay attention to medications that have been effective in the past | |
Quality | • Children can be surprisingly clear in their descriptions of what the pain feels like |
• An intense, well-localized pain can suggest bone pain | |
• Neuropathic pain can be described as numbness, burning, paraesthesia or hyperaesthesia (change of sensation) | |
Radiation | • Neuropathic pain is typically distributed in a recognizably dermatome pattern |
Severity | • Use age-appropriate pain measurement tools |
• Record the measurements in the child’s clinical charts or journal (kept by the child or his/her parents) | |
Timing | • Neuropathic and bone pain are usually constant in nature |
• Causes of intermittent pain: | |
– Cause is episodic in nature, e.g. intestinal colic due to constipation, stimulant laxatives, or pathological fracture that is only painful when it is moved | |
• Management of these differ and it is important to distinguish between them |
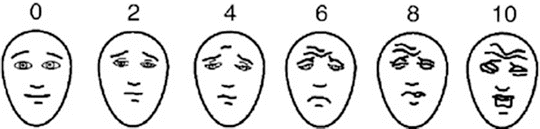
A challenge in assessing pain in children is that many children are non-verbal and therefore cannot directly report their pain. Even young children, who are verbal, might not understand the standard 0–10 numeric scale used in adults. A number of pain assessment scales have been developed for young children and non-verbal children. These include the CRIES scale for infants (Crying, Requires oxygen, Increased vital signs, Expression, Sleeplessness), the FLACC scale for very young and non-verbal children (Face, Legs, Activity, Cry Consolability), the Faces Pain Scale-Revised for children 3–8 years old and the standard numeric scale for older children and adolescents [20]. Figure 11.2 illustrates the Faces Pain Scale-Revised (Bieri Faces scale), one of the most frequently used measures of pain in young children.
Pain management. Like all aspects of medical care pain management requires a systematic and analytical approach. The approach must be sensible and realistic, with careful attention to the principles and evidence that support our understanding of the pathophysiology of pain [12]. Optimal pain management requires a comprehensive approach comprising judicious use of non-opioid, opioid analgesics, adjuvants and non-pharmacological strategies. Such an approach is possible in resource-limited settings [8].
The World Health Organization (WHO) guidelines on the pharmacological treatment of persistent pain in children with medical illnesses have become the gold standard in the pharmacological treatment of persistent pain, including cancer pain. In the new guidelines (2012) the WHO recommends using analgesic treatments in two steps (see Table 11.3) [4].
Table 11.3




WHO guidelines [4]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree