Despite significant advances in our understanding of the microbiology and pathogenesis of pelvic and intra-abdominal infections and substantial improvement in the diagnosis and treatment of these infections, soft tissue pelvic/intraabdominal infection remains a major source of morbidity and mortality on obstetrical and gynecological services.
In the preantibiotic era, obstetrician-gynecologists were primarily concerned with the group A β-hemolytic streptococcus (Streptococcus pyogenes), which in that era was a leading cause of maternal mortality. The 1950s saw the emergence of Staphylococcus aureus and with it the first major concern over antibiotic resistance. In the 1960s, the focus turned on Gram-negative Enterobacteriaceae, especially Escherichia coli and the Gram-positive anaerobic rod Clostridium perfringens.
Commencing in the 1970s, the important role of anaerobic bacteria especially Gram-negative anaerobic rods such as Bacteroides fragilis became apparent. The following decade saw the emergence of anaerobes such as Prevotella bivia and Prevotella disiens (formerly Bacteroides bivius and Bacteroides disiens) and black-pigmented Gram-negative anaerobes in obstetric and gynecologic infections.
Recognition of the critical role played by anaerobic bacteria in obstetric and gynecologic infections resulted in the concept of mixed anaerobic and aerobic infection as the predominant form of pelvic (as well as intra-abdominal) infection
(Box 7.1). Thus, most infections of the female upper genital tract are polymicrobic and arecaused by mixtures of anaerobic and aerobic (facultative) bacteria.
Also during this time, the Group B Streptococcus emerged as a major pathogen in neonatal sepsis and maternal infectious morbidity and clinicians recognized the importance of
Chlamydia trachomatis in infections of the genital tract. Subsequently, in the late 1980s and early 1990s the importance of the Enterococcus in pelvic and intra-abdominal infections became apparent. Most recently, the emergence of methicillin-resistant
S. aureus (MRSA) as a pathogen in obstetric and gynecologic patients has been noted. Fortunately, Pseudomonas aeruginosa remains an uncommon pathogen in obstetric and gynecologic patients and multidrug resistant Enterococcus has not yet affected obstetric and gynecologic services.
Table 7.1 provides a schematic depiction of the common pathogenic bacteria involved in infections of the female upper genital tract and intra-abdominal cavity.
A wide variety of soft tissue infections of the female upper genital tract occur. These include:
1. Obstetrical infections (intra-amniotic infection, chorioamnionitis, postpartum endomyometritis)
2. Postgynecologic surgical procedure (cuff cellulitis, cuff abscess, peritonitis, pelvic abscess)
3. Pelvic inflammatory disease (endometritis, salpingitis, pyosalpinx, tubo-ovarian abscess).
To prevent significant morbidity or potential mortality, the obstetrician-gynecologist must develop appropriate management plans for the treatment of soft tissue pelvic infections. These plans must be based on knowledge of the microorganisms involved, the microbiologic techniques capable of isolating the mixed anaerobic and facultative bacteria present, knowledge of the available antibiotics and their spectrum of activity, an awareness of the potential side effects of these antibiotics and, last, a realization that surgical intervention may be necessary to eradicate the infection. Furthermore, because mixed infections with multiple species of aerobic and anaerobic bacteria involve a complex flora that precludes rapid identification of the pathogens involved in these infections, therapy of most pelvic infections must be empiric.
ETIOLOGY AND PATHOGENESIS OF SOFT TISSUE PELVIC INFECTIONS
Two basic underlying principles are crucial to an understanding of the pathogenesis of female genital tract infections. The first of these is that except for a few micro-organisms such as the group A β-hemolytic streptococcus, Neisseria gonorrhoeae, and C. trachomatis, the pathogens that cause upper genital tract infections in the pelvis arise from the normal microflora of the vagina and cervix. The lower genital tract of healthy women is a complex ecosystem that harbors multiple bacterial species, both anaerobic, aerobic, and facultative bacteria. An average number of 6 to 7 organisms reside in the vagina and cervix and are present in concentrations of 108 to 109 bacterial per milliliter. In this milieu, anaerobes are the most prevalent organisms and outnumber the facultative bacteria by a factor of 10 to 1. The anaerobic bacteria commonly identified in the normal microflora of the vagina and cervix include lactobacilli; anaerobic Gram-positive cocci (Peptostreptococcus); and anaerobic Gram-negative rods (Prevotella species, P. bivia, and P. disiens) and black pigmented Gram-negative rods. The B. fragilis group of organisms are present to a lesser degree. The most common facultative bacteria of the normal vagina and cervix appear to be lactobacilli, aerobic streptococci, Staphylococcus epidermidis, and Gardnerella vaginalis. E. coli is recovered in 5% to 30% of healthy female lower genital tracts; other Enterobacteriaceae are generally found in less than 10% of the population.
The second guiding principle is the recognition that Pasteur and Koch’s theorem that a single pathogen is responsible for a single infection no longer holds. Rather, identification of the presence in pelvic infections of a complex microflora that includes multiple aerobic and anaerobic bacteria has led to the so-called polymicrobic etiology of infection. In general, anaerobic bacteria are recovered from nearly two thirds of pelvic infections and are the only isolates in approximately one third of such infections. It has become apparent that anaerobic bacteria are probable pathogens in virtually all types of bacterial infections of the female pelvis. Many investigations demonstrated that B. fragilis was both a frequent and major pathogen in soft tissue pelvic infections. More recent studies have shown that unlike intra-abdominal infections in which B. fragilis has remained the anaerobe most commonly isolated, this organism is not the most frequent anaerobe identified in pelvic soft tissue infections (<5% of infections). Rather, recent studies demonstrate the importance of P bivia and P disiens, which have been recovered from pelvic infections in 19% to 44% and 2% to 16% of patients, respectively, and black pigmented Gram-negative anaerobes.
Alterations in the normal environment resulting from medical therapy or operative intervention can result in conditions appropriate for selective anaerobic survival and proliferation. A variety of factors facilitate the access of the normal microflora of the cervix and vagina to the upper genital tract and allow the appropriate environmental conditions for the organisms to act as pathogens producing clinical infection. These factors include damage to mucosal surfaces, impairment of local vascular supply, presence of traumatized or necrotic tissue, the presence of foreign bodies, and the growth of exogenous organisms (e.g., N. gonorrhoeae and C. trachomatis) which produce tissue destruction.
MANAGEMENT OF SOFT-TISSUE PELVIC INFECTIONS
Selection of antimicrobial agents for the treatment of pelvic infections is based on knowledge of the micro-organisms usually involved, the nature and severity of the infection, and the susceptibility patterns of available antimicrobial agents. In addition, cost of treatment must be taken into account, but only when efficacy and safety are equivalent. It is now widely accepted (as described above) that pelvic infections are characteristically caused by mixtures of anaerobic bacteria, especially Peptostreptococcus species, B. fragilis group, Prevotella species, P. bivia, P. disiens and black pigmented Gram-negative anaerobic rods; facultative Gram-negative Enterobacteriaceae such as E. coli; and facultative streptococci. In patients with acute pelvic inflammatory disease (PID), N. gonorrhoeae and/or C. trachomatis are frequent pathogens either with or without mixed anaerobic and aerobic bacteria.
In addition to recognizing the susceptibility patterns of the potential pathogens, several other factors are useful in determining the initial selection of antimicrobial therapy for these mixed pelvic infections. It is crucial that the antimicrobial agent reach the infected space, such as an abscess, soft tissue spaces of the pelvis, or amniotic fluid. The efficacy of new antimicrobial agents can be assessed in experimental animal models. However, ultimately the efficacy of antibiotics must be compared in prospective randomized clinical trials.
Experimental models of intra-abdominal sepsis have enhanced both our knowledge of the pathogenesis and microbiology of these infections and their therapy. The intra-abdominal sepsis model of Weinstein et al is an excellent description of the pathogenesis of mixed aerobic-anaerobic infections of the abdomen and pelvis
(Fig. 7.1). The initial stage of peritonitis, sepsis, and an associated high mortality rate of approximately 40% appears to be caused by Gram-negative facultative bacteria, especially
E. coli. The surviving animals over the next several days develop a secondary phase characterized by the development
of intra-abdominal abscesses. The microorganisms associated with these abscesses are anaerobes, in particular,
B. fragilis. A similar biphasic disease process occurs in many clinical entities that are encountered in obstetrics and gynecology. Whereas pelvic inflammatory disease, pelvic cellulites, and endomyometritis are analogous to the initial phase of peritonitis in this model, these infections, if untreated or inadequately treated, will progress to an abscess stage characterized by the presence of entities such as pyosalpinx, tubo-ovarian abscess, or pelvic abscess.
The Weinstein animal model has also been used in an attempt to identify the appropriate management for mixed infections. With the recognition that we were dealing with multiple organisms, both aerobes and anaerobes, it became crucial to determine whether or not the majority of organisms recovered from the site of infections must be treated, or whether only particular agents require therapy. In this animal model, it became apparent that although treatment of the peritonitis stage with agents effective against only the Gram-negative facultative bacteria such as
E. coli would prevent the initial stage of peritonitis and sepsis, it had almost no effect on the subsequent development of abscesses
(Table 7.2). On the other hand, with agents only effective against anaerobic bacteria such as clindamycin or metronidazole, although the abscess stage was prevented, the animals universally developed peritonitis with a high mortality rate. It became apparent that either combination therapy with agents effective against both anaerobic bacteria and Gram-negative facultative bacteria, or single agents effective against both components, was necessary to prevent peritonitis, sepsis and its associated high mortality, and the development of intra-abdominal abscesses.
This basic tenet of utilizing combination or single agent therapy that is effective against the resistant Gram-negative anaerobes such as B. fragilis, P. bivius, and P. disiens early in the disease process has been demonstrated to be appropriate and applicable to the clinical situation as well. A 1979 report for LA County Hospital was a bellweather study which clearly identified the benefits of this early aggressive approach in the therapy against resistant anaerobes. Those patients initially receiving an agent effective against B. fragilis (clindamycin) had significantly less morbidity, required less additional antibiotics or surgical intervention, had less serious infection complications such as abscesses or septic pelvic thrombophlebitis, and spent a mean of 1.6 less day sin the hospital than those women receiving an agent (penicillin) that did not cover the anaerobic Gram-negative rods of the Bacteroides group. As a result, the approach to clinical management of soft tissue pelvic infections changed dramatically. Prior to this report, the traditional approach in obstetric and gynecologic infections had been to initiate treatment with ampicillin or a first generation cephalosporin alone or in combination with gentamicin. Anti-anaerobic drugs such as clindamycin, chloramphenicol or metronidazole were reserved for patients not responding to initial therapy. Following publication of these results, early aggressive initial treatment with a regimen including an agent effective against Prevotella species and B. fragilis became the standard of care.
Table 7.3 is an attempt to simplify the approach to managing pelvic infections by organizing the pathogenic organisms into five major groups according to antimicrobial susceptibility patterns.
Because of their frequency in pelvic infections, clinicians should assume that Gram-negative anaerobic rods are present whenever an infection of the female upper genital tract occurs and their choice of an antimicrobial regimen should include coverage for these resistant β-lactamase-producing anaerobes.
Antibiotic-resistant anaerobic bacteria have become increasingly recognized as a factor that must be taken into account in the selection of antimicrobial agents. Antibiotic resistance among anaerobes has steadily increased over the past several decades. B. fragilis is the most frequently isolated antibiotic resistant anaerobe. Both retrospective and prospective studies have demonstrated an association between antibiotic susceptibility among anaerobic bacteria, especially B. fragilis, and adverse outcomes.
Although clindamycin has been the gold standard for treatment of anaerobic infection since the 1970s, antibiotic resistance to clindamycin among the B. fragilis group in national surveys has steadily increased from 3% in 1987 to 16% in 1996 and 26% in 2000. As a result of this rapid increase in clindamycin resistance, this agent is no longer considered by many experts to be first-line therapy against the B. fragilis group. Resistance to clindamycin amongst other anaerobes (e.g., Prevotella, Porphymonas, Peptostreptococcus, and Fusobacterium) is generally <10%.
Greater than 97% of the
B. fragilis group organisms are resistant to penicillin G. Although cefoxitin and cefotetan have much better activity against
B. fragilis group organisms, the prevalence of resistance to those agents has also increased. In 1987 to 2000, resistance to cefoxitin was seen in 8% to 14% of isolates. Cefotetan has similar activity to cefoxitin for
B. fragilis but is much less active against other members of the
B. fragilis group. Similarly, piperacillin resistance has also increased with
up to 25% resistance to the
B. fragilis group recently being noted. As a consequence, these agents are generally not recommended as empirical single agent therapy for moderate-to-severe pelvic/intra-abdominal infections.
Resistance among Gram-negative anaerobic bacteria to metronidazole is rare. There have been no reported resistant strains reported in the United States. However, resistant strains have been reported worldwide. Metronidazole resistance is more common among Gram-positive anaerobes, especially Peptostreptococcus and Actinomyces species.
The most active β-lactam antimicrobial agents against the B. fragilis group organisms are β-lactam/β-lactamase inhibitor combination (e.g., ampicillin/sulbactam, ticarcillin/clavulanate, piperacillin/tazobactam) with <2% of B. fragilis group organisms resistant in a 2000 national survey.
In evaluating the therapeutic response of obstetric and gynecologic infections, several factors must be taken into account. Not only is the patient’s initial response to antimicrobial therapy crucial, but we must also evaluate the need for additional antimicrobial agents, the need for use of anticoagulants for the treatment of septic pelvic thrombophlebitis and, most importantly, the need for surgical intervention for either drainage or surgical extirpation of abscesses or infected tissues
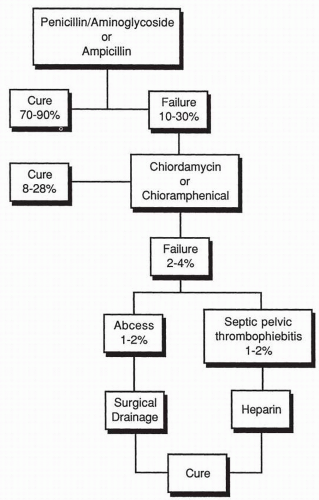
(Box 7.2).A schematic approach to the therapy of mixed aerobic-anaerobic pelvic infections is depicted in
Figure 7.2. The traditional
approach to the management of these infections was the use of penicillin-aminoglycoside regimen, ampicillin by itself, or a first-generation cephalosporin by itself. With such antimicrobial regimens, cure rates were obtained in 70% to 90% of patients. Those patients not responding within an appropriate period of time were then treated with clindamycin or chloramphenicol for supposed
B. fragilis infection. Once again, the vast majority of patients would respond, but there consistently remained a small number of failures, of which 1% to 2% had pelvic abscesses requiring surgical drainage, and 1% to 2% developed septic pelvic thrombophlebitis requiring anticoagulation therapy with heparin. More recently, a more aggressive approach has been used in which therapy is commenced with agents effective against
B. fragilis and the
Prevotella group of bacteria rather than waiting for the patient to fail to respond. It is inappropriate to expose 10% to 30% of patients with pelvic infections to potential life-threatening complications such as abscess formation and septic pelvic thrombophlebitis.
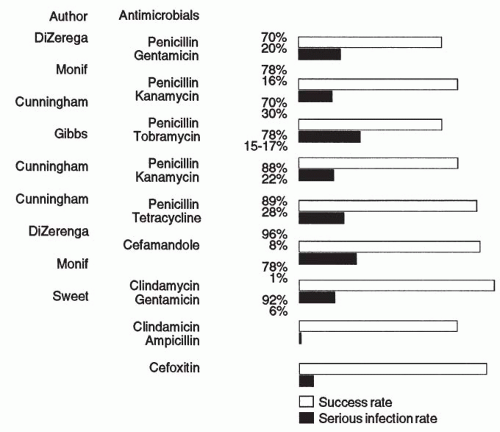
Studies of pelvic infection that used antimicrobials that did not provide coverage for
B. fragilis or other resistant anaerobes of the
Prevotella species have reported cure rates ranging from 70% to 90% but noted that these regimens result in a 5% to 29% occurrence rate of severe infections (i.e., pelvic abscess, wound abscess, and/or septic pelvic thrombophlebitis). On the other hand, studies that included antimicrobial regimens effective against resistant anaerobes such as
B. fragilis and
B. bivius reported cure rates in the 87% to 100% range and, most significantly, a lower incidence of severe infections, ranging from 0% to 15%
(Fig. 7.3).A variety of antimicrobial combinations and single antimicrobial agents provide effective therapy against the anaerobic bacteria, including
B. fragilis and members of the
Prevotella species and many of the facultative bacteria associated with the infections of the female genital tract
(Table 7.4). These include either clindamycin or metronidazole in combination with an aminoglycoside with or without ampicillin. Aminoglycosides have been the traditional choice for coverage of aerobic Gram-negative bacilli in soft tissue pelvic infections when combination therapy is chosen. Recent, once-daily aminogly-coside dosing has been proposed as an alternative to the traditional multiple dosing per day. This approach is based on two features of aminoglycosides: concentration-dependent bactericidal activity and a long postantibiotic effect (up to 7.5 hours). This allows for a prolonged dosage interval during which bacterial regrowth dose not occur despite serum levels of the drug below the MIC. The new third-generation cephalosporins or aztreonam could be used in place of the aminoglycoside.
A variety of single agents are available with demonstrated efficacy in the treatment of mixed anaerobic-aerobic pelvic infections. These include the cephamycins (cefoxitin and cefotetan); possibly the third-generation cephalosporin, ceftizoxime; the extended-spectrum penicillins (mezlocillin and piperacillin); the carbapenems (imipenem, meropenem, and ertopenem); and the β-lactam plus enzyme blockers (sulbactam/ampicillin [Unasyn], ticarcillin/clavulanic acid [Timentin], and piperacillin/tazobactam [Zosyn]). For pelvic infections, it would generally be unnecessary to add an aminoglycoside to these agents because of the paucity of P. aeruginosa as a pathogen in nonimmunosuppressed obstetric and gynecologic patients. Carbapenems are probably the optimum single agent for treatment of soft tissue pelvic infections as they have the broadest spectrum of activity including some Enterococcus and Pseudomonas aeruginosa. However, they generally are not used as a first line drug but rather reserved for severe infections.
In approximately 1% of pelvic infections, patients fail to respond to appropriate antimicrobial therapy and do not have an abscess or hematoma requiring surgical intervention. In these patients, a diagnosis of septic pelvic thrombophlebitis should be considered. Heparin therapy is commenced (antimicrobial therapy is continued) as both a diagnostic and therapeutic tool. The goal is to achieve a partial thromboplastin time (PTT) that is 2.5 times control. If the diagnosis is correct, the patient should rapidly respond and become afebrile within 24 to 36 hours. Heparin therapy is continued for 10 days, unless septic pulmonary emboli occur. In this circumstance, long-term anticoagulation is necessary.
At times, the best antimicrobial agent for the management of pelvic infections is the competent surgeon who applies prophylactic antibiotics properly, uses good surgical technique, and uses surgical drainage and excision of necrotic tissue where appropriate. The clinician caring for women with pelvic infections must recognize that often, surgical intervention is the critical factor for resolution of pelvic soft tissue infection associated with mixed anaerobic-facultative bacteria.