INTRODUCTION
Small cell lung cancer (SCLC) is an aggressive bronchogenic carcinoma diagnosed in 14% of all patients with lung cancer, accounting for approximately 30,000 new cases annually in the United States (1). It is distinguished from non–small cell lung cancer (NSCLC) by its rapid doubling time, high proliferative fraction, and early development of metastases. Regional lymph node involvement or distant metastasis is present in 90% or more of patients at diagnosis. Historically, SCLC has been staged as limited disease (LD), which is confined to the ipsilateral thorax of origin and regional nodes, versus extensive disease (ED). The recent International Association for the Study of Lung Cancer (IASLC) staging project and American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) seventh edition suggest use of the tumor, node, metastasis (TNM) system for the staging of SCLC (2). Clinically, the limited- and extensive-stage classification is practical given that most patients present with advanced disease (stages III-IV) and are only rarely candidates for resection.
Standard treatment for LD (stages I-IIIB) includes both chemotherapy and radiation; chemotherapy is the mainstay of treatment for ED (stage IV). Although a dramatic response to initial therapy is usually observed, greater than 95% of patients with ED and 80% to 90% of those with LD eventually suffer relapse and die of their disease.
Despite extensive research, no substantive advances in the systemic treatment of SCLC have been made for decades. Molecular profiling and preclinical models of SCLC have increased our understanding of the biology and genomic changes in the pathogenesis of SCLC. Translation of preclinical research to the clinical arena has resulted in recent promising data with targeted therapies, providing hope that improved outcomes for patients is on the horizon.
EPIDEMIOLOGY
Small cell lung cancer is uncommon in never smokers, who constitute only 3% to 5% of cases, and is commonly associated with intense tobacco exposure (3). However, transformation to SCLC has been recently documented in never smokers with epidermal growth factor receptor (EGFR)–mutation positive adenocarcinoma of the lung, in the setting of resistance to tyrosine kinase inhibitors (4). The original EGFR mutation is maintained in the SCLC, supporting the notion that the tumor evolved from transformation and is not a second primary cancer.
The incidence of SCLC has steadily declined, as illustrated by an analysis of the Surveillance, Epidemiology, and End Results (SEER) database (1), in which the proportion of SCLCs decreased from 17% in 1986 to 13% in 2002. However, this decrease was accompanied by an increase in SCLC cases arising in women (28% in 1973 vs 50% in 2002), attributed to increasing tobacco use among women starting in the 1960s. The reduced incidence may be related, in part, to changes in the pathologic criteria leading to the classification of cases as large cell neuroendocrine carcinoma (LCNEC) that would have been previously classified as SCLC.
RISK FACTORS
Of all lung cancer subtypes, SCLC shows the strongest association with tobacco exposure, which represents the most important risk factor (3). The risk is related to both the duration (>40 years) and intensity of tobacco use (>30 cigarettes/day). This risk is lower in former smokers versus current smokers, although the risk in former smokers still exceeds that of nonsmokers (3). Additional risk factors include exposure to asbestos, benzene, coal tar, and radon gas, usually as cocarcinogens with tobacco. Smoking cessation should be encouraged as a method of primary prevention. Patients with LD who continue to smoke during or after chemoradiation experience increased toxicity, have a high risk of second lung cancers, and have shorter survival than those who quit (5).
NATURAL HISTORY
The natural history of SCLC was documented in the placebo arm of a randomized trial from the Veterans Administration Lung Cancer Study Group (VALSG) reported in 1969, testing the effect of three doses of intravenous cyclophosphamide (6). In this trial, the median survival for patients in the placebo arm was 6 weeks for those with ED and 12 weeks for those with LD, based on primitive staging studies at that time. Due to stage shift with modern staging techniques, outcomes in both groups would be likely better today. Cyclophosphamide increased the median survival by 75 days in both groups, tripling the survival of patients with metastases and doubling that of patients with LD. This was the first observation foretelling the important role chemotherapy would come to play in management of SCLC.
The use of effective combination chemotherapy and, in the case of patients with tumor amenable to definitive radiation, the use of multimodality treatment have improved survival of SCLC patients. For patients with LD, 5-year survival was less than 5% in 1973 and improved to 10% in 2000. In the same period of time, 2-year survival for patients with ED improved from 1.5% to 4.6% (1).
PROGNOSTIC FACTORS
The most important prognostic factor for SCLC is the stage, as patients with LD have improved survival compared with those with ED (7). Among patients with LD, good performance status (PS), age less than 70, female gender, and normal lactate dehydrogenase (LDH) are predictive of a favorable outcome (7). Among these patients, a small subgroup with very LD (no mediastinal involvement) was found to have a longer median survival when treated with surgery (8). In patients with ED, normal LDH, multidrug regimen treatment, and a single metastasis predicted better outcomes. Liver or cerebral metastases confer significantly shorter survival compared to bone, soft tissue, or bone marrow involvement (9). Paraneoplastic syndromes (PNSs) may also predict outcome. Patients with the syndrome of ectopic corticotrophin (ACTH) secretion producing clinical Cushing syndrome have a dismal prognosis, with a low response to chemotherapy and poor control of hypercortisolism following treatment (7). Lambert-Eaton myasthenic syndrome (LEMS), an autoimmune PNS, confers a more favorable prognosis, presumably due to immunity against the cancer (10).
PATHOBIOLOGY
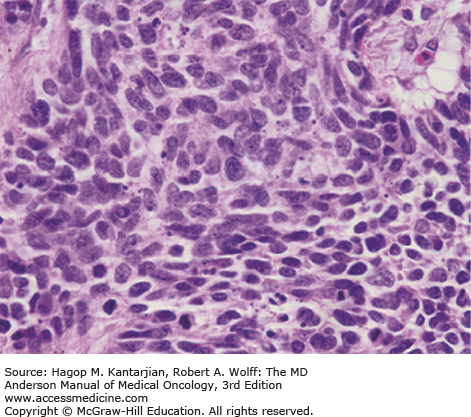
Small cell lung cancer is defined by light microscopy as a malignant epithelial tumor consisting of small cells, with round-to-fusiform shape, scant cytoplasm, finely granular nuclear chromatin, and absent or inconspicuous nucleoli (11). Nuclear molding and necrosis are frequent, and mitotic rates are high (Fig. 17-1). Tumors usually grow in diffuse sheets, but rosettes, peripheral palisading, organoid nesting, streams, ribbons, and rarely, tubules or ductules may be present. Typically, diagnosis is made from small biopsies and cytology specimens, as surgery is rarely performed. Due to significant crush artifact, biopsies are sometimes more problematic in diagnosis than cytology specimens.
Combined small and large cell carcinoma is histologically a tumor with a mixture of SCLC and at least 10% larger cells that morphologically fall under the definition of NSCLC. Additional variants exist, including combined SCLC with squamous cell, adenocarcinoma, spindle cell, or giant cell carcinoma. The frequency of combined SCLC varies according to tumor sample size, number of histological sections analyzed, type of specimen, and interpretation. SCLC is a “small, round, blue cell tumor” using a hematoxylin and eosin (H&E) stain, and the differential diagnosis includes other small, round, blue cell tumors, including lymphomas and small cell sarcomas. Histologically, identical tumors can arise in other organs (eg, nasopharynx, larynx, genitourinary or gastrointestinal tract, and cervix) and are termed extrapulmonary small cell carcinomas. Both pulmonary and extrapulmonary small cell carcinomas have similar biological features and clinical behavior, with high potential for widespread disease. However, malignant cells from extrapulmonary small cell carcinomas do not exhibit 3p deletions, which are common in SCLCs, indicating, at least in part, differences in carcinogenesis (12).
Immunohistochemical (IHC) markers are valuable in differential diagnosis of SCLC. Positive pancytokeratin (AE1/AE3) staining helps to identify the tumor as a carcinoma rather than a lymphoma or sarcoma (11). Neural cell adhesion molecule (CD56), chromogranin, and synaptophysin are the most useful markers. While CD56 expression is detectable in approximately 90% to 100% of cases, SCLC may be negative for expression of neuroendocrine markers, such as chromogranin and synaptophysin (13). Small cell lung cancer (SCLC) is a primitive undifferentiated high-grade neuroendocrine tumor (NET) and does not typically express these proteins as intensely as low-grade, well-differentiated NETs do, such as carcinoids. In 10% of cases, all neuroendocrine markers may be negative, and the diagnosis can still be established if the morphology is diagnostic. Thyroid transcriptase factor 1 (TTF-1) is expressed in 70% to 90% of SCLCs; however, this marker may also be expressed in extrapulmonary small cell carcinomas and thus does not reflect lung origin (14). The Ki-67 staining index, reflecting proliferation, is generally greater than 50% in SCLC and can be used to differentiate SCLC from lower-grade NETs (15).
GENOMIC AND PROTEOMIC ALTERATIONS
Small cell lung cancer is characterized by genomic alterations, biology, and clinical behavior that are distinct from the intermediate- and low-grade pulmonary NETs. It appears that SCLC is driven more by mutations and deletions of tumor suppressor genes than by alterations in oncogenes. Loss of function of tumor protein 53 (TP53) occurs in 75% to 90% of SCLCs (16). Loss of the retinoblastoma 1 (RB1) gene at 13q14 occurs in virtually all patients with SCLC (16). Haploinsufficiency due to allele loss in multiple areas on chromosome 3p, including 3p21.3, 3p12, 3p14.2, and 3p24.4, leads to absent or lower expression of several tumor-suppressor genes in greater than 90% of SCLCs and is an early event in tumorigenesis (12). Deletion of the TGFBR2 gene, encoding the transforming growth factor beta type II receptor, has been described in SCLC (17). The tumor suppressor gene FUS1, in the 3p21.3 region, was not expressed in 100% of SCLCs examined in one series (18). RASSF1A encodes a protein involved in cell cycle, apoptosis, and microtubule stability and is inactivated in 90% or more of SCLC (19).
Cells may acquire immortality by compensating for the loss of telomeric repeats through telomerase reactivation. Telomerase RNA subunit (hTR) and telomerase activity are upregulated in 98% or more of SCLC (20). Increased expression of cKit, and its ligand stem cell factor, is detected in up to 80% to 90% of SCLCs (21). Amplification of MYC family members (v-myc avian myelocytomatosis viral oncogene homolog, MYC, MYCL1, and MYCN) is detected in 20% of SCLCs (16). Loss of phosphatase and tensin homolog (PTEN) is observed in 2% to 4% of tumors (20); however, the Phosphoinositide 3-kinase (PTEN) pathway alteration rate(PI3K) pathway alteration rate may be overall higher and may promote SCLC tumorigenesis in preclinical models. The BCL-2 family proteins exert an antiapoptotic effect and may be upregulated in 75% to 95% of SCLC (16).
Two comprehensive genomic profilings of SCLC confirmed common DNA alterations and their relation with tobacco exposure (22) and identified novel potential therapeutic targets, including SOX2 (sex-determining region Y box 2) amplifications and RLF-MYCL1 fusions (23). Proteomic profiling has identified differences in protein expression between SCLC or LCNEC and other NSCLC cancers, including the DNA repair protein poly(ADP-ribose) polymerase 1 (PARP1), checkpoint kinase 1 (Chk1), and chromatin modulator enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) (24). Table 17-1 describes common genomic and proteomic alterations that occur in SCLC.
| Genes | Mutation Frequency (Type of Mutation) |
|---|---|
| TP53 | 75-90% Loss of function (mutation, LOH, deletion) |
| RB1 | ~100% Loss of function (mutation, LOH, deletion) |
| PTEN | ~5% Loss of function (mutation, LOH, deletion) |
| MYC | 18-31% MYC family alterations overall Gain of function (amplification or transcrptional dysregulation) |
| SOX2 | 27% Gain of function (amplification) |
| FGFR1 | <10% Gain of function (amplification, mutation) |
| CCNE1 | <10% Gain of function (amplification) |
| EPHA7 | <10% Gain of function (amplification) |
| PARP1 | >50% (overexpression of protein target) |
CLINICAL PRESENTATION
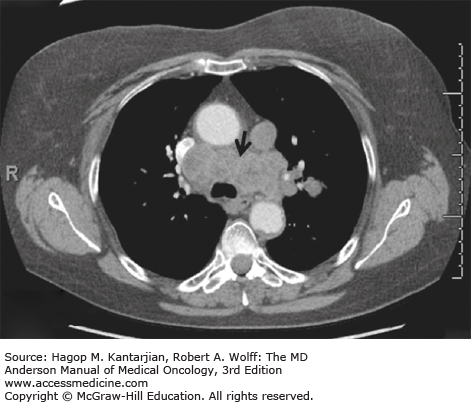
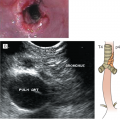
Small cell lung cancer typically arises in the central airways and infiltrates the submucosa, with a tendency to narrow the bronchial lumen through extrinsic or endobronchial spread, in contrast to squamous cell carcinomas, where polypoid luminal occlusion is common. Rapid intrathoracic tumor growth, lymphatic and distant spread, and manifestation of PNSs can cause severe and progressive symptoms that lead to diagnosis generally within 3 months from onset. Common clinical manifestations include cough, dyspnea, weight loss, and debility. Hemoptysis and postobstructive pneumonia are relatively uncommon due to the submucosal growth pattern of the tumor. Bulky involvement of mediastinal lymph nodes is a hallmark of SCLC, and syndromes resulting from mass effects are commonly seen, including superior vena cava syndrome, hoarseness (from recurrent laryngeal nerve compression), phrenic nerve palsy, dysphagia (from esophageal compression), and stridor (from tracheal compression). Small cell lung cancer is the most common malignant cause of superior vena cava obstruction. Radiographically, a large hilar mass with bulky mediastinal adenopathy is commonly observed (Fig. 17-2), although occasional peripheral satellite nodules may be found.
Most patients present with overt metastatic disease (60%-70%); common sites of metastasis include liver, adrenals, bone, bone marrow, and brain. Symptoms of metastatic disease can include bone or right upper quadrant abdominal pain, headache, seizures, fatigue, and anorexia. Occasionally, patients may present with PNSs, such as syndrome of inappropriate antidiuretic hormone (SIADH) or LEMS.
STAGING
The two-stage system originally proposed by the VALSG in the late 1950s and later modified by the IASLC is currently used for simplicity and practicality. Limited disease (LD) is defined as a tumor confined to one hemithorax, with involvement of regional nodes, including contralateral mediastinal or ipsilateral supraclavicular nodes, that can be included in a single tolerable radiotherapy (RT) port (TNM stages I-IIIB). Extensive disease (ED) is defined as a tumor beyond the boundaries of LD, including distant metastases, malignant pericardial or pleural effusions, and contralateral supraclavicular and contralateral hilar node involvement (TNM stage IV, any T, any N, M1a/b). More recently, IASLC proposed changes to the TNM NSCLC staging system, mainly in T and M descriptors and stage groupings. These changes have been incorporated into the AJCC seventh edition, which recommends TNM staging for SCLC and NSCLC (2). However, the two-stage system continues to be used in clinical practice, as most patients present with advanced disease (stages III-IV) and are only rarely candidates for surgery. This system has prognostic significance and clinical implications because patients with LD are candidates for chemoradiation with curative intent, while patients with ED receive chemotherapy alone and palliative radiation as clinically indicated. Approximately 30% to 40% of patients with SCLC present with LD; the remainder will have ED (1).
The initial clinical evaluation should include history and physical examination, chest radiograph, pathology review, baseline laboratory tests, including complete blood cell count, a comprehensive metabolic profile, LDH measurement, computed tomographic (CT) scans of the chest and abdomen with intravenous contrast, and brain magnetic resonance imaging (MRI) or CT scan with intravenous contrast. Brain MRI is more sensitive than CT in identifying metastases and is preferred. If LD is suspected, a positron emission tomography (PET)–CT scan may be indicated because it can identify distant disease and guide mediastinal evaluation. For most metastatic sites, a PET-CT scan is superior to other imaging modalities; however, it is inferior to MRI or CT of the brain for detection of brain metastases. Despite the fact that PET-CT may improve staging accuracy of SCLC, pathologic confirmation is still recommended for lesions depicted at PET-CT scan that may alter staging. If PET-CT scan is not available or is equivocal, bone imaging with a whole-body bone scan should be used to stage the skeleton.
Staging should not focus only on symptomatic sites of disease or on altered laboratory data. For example, bone scans may be positive in up to 30% of asymptomatic patients without abnormal alkaline phosphatase. The goal of complete staging is to identify those patients with LD who are candidates for definitive therapy. In the presence of obvious ED, staging may be clinically directed. Brain imaging should be obtained in all patients, given the morbidity of uncontrolled central nervous system (CNS) disease. Imaging of the CNS reveals brain metastases in 10% to 15% of patients at diagnosis, with 30% of these individuals asymptomatic.
In patients with apparent stage I disease (T1-2N0) surgical resection should be considered if mediastinal staging is negative (8). This can be obtained by conventional mediastinoscopy, transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), or video-assisted thoracoscopy (VATS). If a pleural effusion is present in a patient with otherwise LD, thoracentesis should be performed and fluid sent for cytology. If the fluid is exudative or if malignant cells are present, the patient should be considered to have ED (stage IV: M1a). While most pleural effusions in lung cancer are related to the malignancy, there may be a few instances in which multiple cytopathological examinations are negative for malignancy and the pleural fluid is not an exudate. When these elements and clinical judgment indicate that a pleural effusion is not malignant, the effusion should be excluded as a staging element. Pericardial effusion is classified according to the same criteria.
Sampling of cerebrospinal fluid is indicated if leptomeningeal spread is suspected. Pulmonary function tests should be performed in patients who are candidates for definitive chemoradiation therapy. Severe anemia, nucleated red blood cells on peripheral smear, neutropenia, or thrombocytopenia, in the absence of other obvious etiologies, are selection criteria for bone marrow aspiration and biopsy. The presence of tumor cells in a marrow biopsy identifies ED. Treatment should be initiated as quickly as possible after diagnosis is confirmed, given the rapid rate of progression. If the patient is significantly symptomatic or the staging evaluation prolonged, staging should be completed while chemotherapy is started.
TREATMENT
Patients with SCLC rarely survive more than a few months without treatment. The disease is generally highly responsive to both chemotherapy and radiation, and patients with LD are treated with curative intent. In LD, the overall response rates (RRs) to combined chemoradiation are typically 80% to 90%, including 50% to 60% complete RRs. Median overall survival (OS) is approximately 17 months, and the 5-year survival rate is approximately 12%.
An autopsy series on patients who died from postoperative complications revealed that 90% of patients with SCLC had mediastinal metastasis within 30 days following surgical resection (25). A randomized trial evaluating surgery versus thoracic RT (TRT) in patients with resectable disease revealed that the TRT group had significantly longer OS (26), suggesting that, even in absence of diagnosed metastases, surgery alone is an inadequate therapeutic strategy. However, surgery may play a role in multimodality therapy for those patients (5%) with early T stage and without nodal involvement (T1-T2N0M0, very LD). A 5-year survival of 48% has been reported in patients with very LD when surgery was followed by adjuvant chemotherapy (8). Adjuvant chemotherapy with four courses of etoposide/cisplatin (EP) should be considered for all patients with surgically resected SCLC. If nodal involvement is found at the time of surgery, chemoradiation and chemotherapy are recommended.
Based on the results of a British Medical Research Council trial demonstrating surgery to be inferior to RT for the treatment of LD (26), TRT became standard of care for local control for these patients. Although early studies indicated that RT could increase local control compared to chemotherapy alone, these studies did not consistently show significant survival benefits to combination therapy over chemotherapy (27). Because of this controversy, randomized trials were performed. Two meta-analyses were performed that showed a significant 2-year survival benefit of 5.4% with the addition of RT to systemic chemotherapy for patients with LD (27,28). It is noteworthy that the best outcomes from these older trials included concurrent as opposed to sequential approaches; in addition, the regimens used in this era were anthracycline- and alkylator-based therapies and were associated with excessive in-field toxicity when administered with concurrent radiation. The development of the EP regimen was critical to improving the tolerance and feasibility of concurrent chemoradiation. Etoposide/cisplatin can be given at full dose with RT, leading to improved disease control.
An anthracycline-based regimen (cyclophosphamide, epirubicin, and vincristine [CEV]) was compared in a phase III trial to EP in patients with SCLC (29). Patients received RT concurrently with the third cycle of chemotherapy, and prophylactic cranial irradiation (PCI) was administered to those who achieved complete remission (CR). The results showed that, in patients with LD, EP was superior to CEV for survival rates at 2 and 5 years (14% and 5% vs 6% and 2%, respectively; P = .0001), as well as median OS (14.5 vs 9.7 months; P = .001). In patients with ED, no survival difference was noted.
The addition of other cytotoxins to the EP regimen—either as a triplet with paclitaxel or alternating therapy with cyclophosphamide, doxorubicin, and vincristine—has also been studied, but to date a new standard in the treatment of LD has not emerged (30,31).
Based on the radiobiology of SCLC, the Intergroup (INT) trial 0096 studied accelerated hyperfractionated RT (AHRT) compared to conventional fractionation in a phase III trial (32). Over 400 patients were randomized between the two arms. All patients received four cycles of EP, and TRT was administered concurrently, starting with the first cycle of chemotherapy. All patients received 45 Gy, either in once-daily 1.8-Gy fractions for 5 weeks or twice-daily 1.5-Gy fractions for 3 weeks. Patients in the twice-daily RT group had an improved median OS (23 vs 19 months) and 5-year survival (26% vs 16%), at the cost of increased weight loss and grade 3 esophagitis (27% vs 11%). Local failure rates were lower in the twice-daily RT arm, presumably the major reason for improved survival.
Intergroup trial 0096 convincingly showed an OS benefit to concurrent chemoradiation with hyperfraction. However, because of concerns for side effects and the logistical difficulties involved in twice-daily treatment, the regimen has not been widely used in the community (33). The control arm of the INT 0096 study used a relatively low total RT dose, and since the results of INT 0096 have become available, conventionally fractionated radiation in higher doses has also been studied. The Cancer and Leukemia Group B (CALGB) trial tested the regimen paclitaxel and topotecan for two cycles, followed by TRT with 70 Gy in 35 daily fractions with concurrent carboplatin/etoposide (CE; total of three cycles) in a phase II study (34). Median OS was 22.4 months, comparable to that found with AHRT in the INT 0096 study.
Another schedule of TRT tested was concomitant boost, in which patients received once-daily radiation through most of their course, then received hyperfractionated therapy at the end of treatment, so that RT was accelerated without the need for twice-daily administration throughout the treatment course. The Radiation Therapy Oncology Group (RTOG) tested this approach in the phase II trial 0239. Patients received 61.2 Gy over 5 weeks, with twice-daily RT in the final 9 days (35). Radiotherapy started on day 1 of chemotherapy with four cycles of EP. Two-year survival rate was 37%, somewhat less than that found in both INT 0096 and the CALGB trial (2-year survival rates of 41% and 48%, respectively).
A prospective INT study of chemoradiation for patients with LD (RTOG 0538/CALGB 30610) is currently active. Originally, this trial included the concomitant boost regimen, but that arm has been dropped after an interim analysis. The trial currently compares 70 Gy once daily with AHRT (45 Gy twice daily) from the INT 0096 study. In both arms, concurrent EP and RT commence with the first cycle of chemotherapy (NCT [National Clinical Trial] 00632853). The primary end points are median OS and 2-year survival rates.
Sequential, concurrent, and alternating chemotherapy have all been tested with TRT. Early studies did not show a survival benefit to concurrent chemotherapy; however, this was likely due to the increased toxicity when using cyclophosphamide- or doxorubicin-based chemotherapy. Etoposide/cisplatin is far better tolerated than these earlier regimens when administered concurrently with RT.
The National Cancer Institute of Canada reported a phase III trial of alternating CAV (cyclophosphamide, anthracycline, vincristine)/EP with TRT in either the second or sixth cycle of chemotherapy (36). The patients receiving early RT experienced improved OS (21.2 vs 16 months). The Japan Clinical Oncology Group (JCOG) compared RT (45 Gy in twice-daily 1.5-Gy fractions) starting with either the first cycle of EP or following completion of the chemotherapy course (37). More myelosuppression was noted in the patients in the concurrent arm, but there was a significant reduction in risk of death for early concurrent therapy, with a hazard ratio (HR) of 0.70 (P = .02). Although not all trials have consistently shown a benefit for concurrent chemoradiation, the data strongly support this and its early integration in treatment when the regimen is EP, as contrasted with anthracycline- or alkylator-based regimens. Furthermore, efficacy outcomes are consistently better with EP in the treatment of LD.
Given that SCLC is such a rapidly dividing malignancy, it has been hypothesized that accelerated proliferation of tumor clonogens can affect outcome, and that treatment should be delivered in a condensed fashion (38). A meta-analysis examined the impact of a novel parameter, time from the start of any treatment to the end of RT, on OS in LD. A shorter SER was found to be a significant predictor of better outcome. For each week that the SER was lengthened, OS at 5 years showed an absolute decrease of almost 2% (38). The data from INT 0096 had a strong influence on this result.
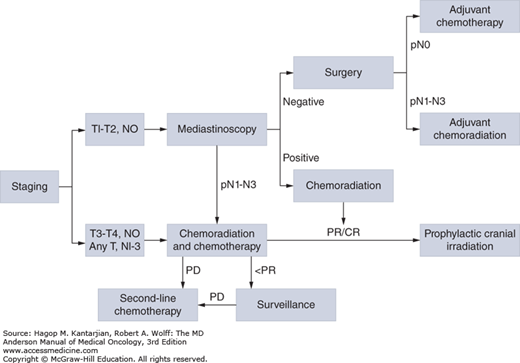
Carboplatin is frequently used in ED due to a more favorable toxicity profile, whereas cisplatin is preferred in patients with LD with curative intent. The efficacy of cisplatin versus carboplatin regimens in LD was evaluated in a meta-analysis of four trials (39), which revealed no significant difference in terms of efficacy. Similar results were found in a subset analysis based on the two randomized trials conducted in patients with LD (40,41), where no differences were seen in RRs or median survival time for EP versus CE, although less toxicity was reported in the carboplatin-containing arm. Nevertheless, due to its use in trials for which the best 5-year survival has been observed in LD, EP should be given unless there is a significant contraindication to cisplatin use. Notably, the dose of cisplatin in INT 0096 was only 60 mg/m2. Figure 17-3 illustrates the treatment algorithm for management of limited-stage SCLC.
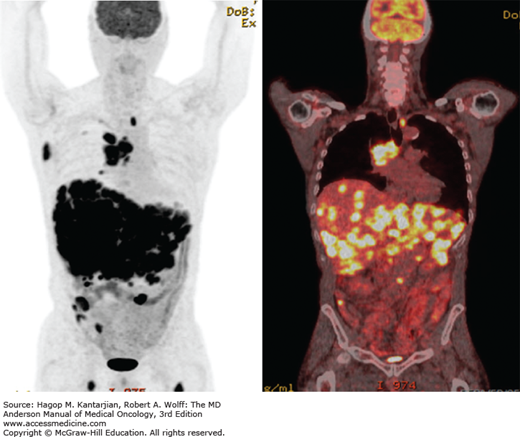
Systemic chemotherapy represents the primary therapeutic modality for patients with ED. Prophylactic cranial irradiation decreases the incidence of symptomatic brain metastases in responders to systemic chemotherapy, although its impact on OS is uncertain. Currently, TRT is utilized for symptom palliation; however, a potential role for radiation in patients with limited systemic disease and a good response to initial therapy has been suggested. Chemotherapy prolongs survival compared with best supportive care (BSC) (6). From the time of diagnosis, the median OS for patients with ED is 8 to 13 months, the 5-year survival is 1% to 2%, and the 2-year survival is 4% to 5% (1). Figure 17-4 shows representative coronal PET-CT images in a patient with extensive-stage SCLC (ED).
In an early study, patients with SCLC had a highly significant improvement in survival with intravenous cyclophosphamide compared to placebo, increasing median survival from 12 weeks to almost 5 months (6). As new cytotoxins became available, combination therapy was studied, and the CAV regimen became the standard of care for ED (29). Etoposide/cisplatin was initially evaluated in patients who had recurrent disease after or failed to respond to CAV, with RRs of 55% (42). It was later evaluated as a first-line treatment in patients unable to tolerate CAV, demonstrating a median OS of 39 weeks in ED, with less toxicity (43). In a phase III trial, the equivalent efficacy of EP and CAV was confirmed by randomizing 437 patients to receive four cycles of EP, six cycles of CAV, or alternation of these two regimens for 18 weeks (CAV/EP, three cycles each) (44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree