Fig. 22.1
Relaxed skin tension lines and biopsy notes
In the end, the surgeon must keep in mind, during planning of the surgical technique chosen, the respect of the integrity of tissues, opting for delicate surgical instruments and which most suite his work and limiting the hemostasis to “needed” (avoiding “the hunt for the red blood cell”) with an adequate intensity that will prevent postoperative hematoma.
Choice for Margins’ Incisions
The surgeon must not under any circumstances choose direct closing of the excision area at the expense of the removal of the entire tumor [18], although there are suspicions of benignity, with adequate margins of safety as prescribed by international oncology protocols [19–24].
The ways in which a plastic surgeon can operate for removal of abnormal growths, malignant or benign, are nowadays numerous. Depending on the anatomical site, the extent of the lesion, and the surgery chosen, the surgeon will have to address and resolve issues concerning the direction of the engraving lines and/or the type of repair to be performed in accordance with the basic principles of plastic surgery. In the following pages, the authors will first describe the basic surgical techniques, then will describe the set of techniques that encompasses advanced surgical procedures as complex as the microsurgical or pedicled flaps, and finally will describe some methods for dressing procedures.
Punch or Circular Scalpel [25]
The use of punch should be relegated to small skin lesions, i.e., not exceeding the maximum size of 8 mm. The resulting gap will have a “round” shape and will be difficult to close through direct suturing. The transition from circular to the oval shape, easier to suture, can be obtained by applying a technical shrewdness during the execution of the incision: the skin incision site should be stretched with two fingers applying forces perpendicularly to relaxed skin tension lines. Once the stretching force exerted is released, the elastic forces of the skin will change the round shape of the punch excision to an oval shape, easier to close and with axis parallel to these skin tension lines. The breach produced by the use of punch can, in alternative and if necessary, be left to heal by secondary intention, as it could result in an acceptable aesthetic result.
Lozenge or Wedge Incision [25]
The lozenge excision is nowadays the most widely used incision to remove skin tumors in all areas of the human body. This surgical practice requires, for all the tension forces involved, matching of the direction of the incision lines to axes coincident or parallel to the course of the relaxed skin tension lines, with dimensions that comply with the ratio of 3–1, i.e., the major axis of the lozenge should be three times the size of the minor axis. The use of this form of excision is necessary for facilitating closure of the breach, free from or almost devoid of tension.
Wedge excisions are generally used if the lesion to be removed is on or adjacent to the free margin of anatomical structures such as eyelids, lips, and ears, allowing for an easy and aesthetically acceptable rebuilding of structures approaching and matching the different tissue planes. Some rules must be followed in carrying out this method with respect to anatomical site: i.e., wedge excision involving the lower lip should never exceed more than 1/3 of the length of the lip itself; moreover, if the excision involves the eyelid, it should never exceed a length equal to 1/4 of the total length of the eyelid; etc.
In order to further reduce tension along the edges of the suture, the surgeon may and sometimes should mobilize widely the margins of the breach itself. The mobilization will be carried out, with few exceptions, within the subcutaneous layer and with respect of the anatomical area: by means of delicate dissecting scissors, mobilization forceps, using scissors stronger than the above but with blunt tips, or even with the use of a finger of a hand.
Then a subcutaneous suture is placed, preferably with braided absorbable suture, which will favor the approach of the edges of the breach so as to minimize possible tension that the skin closure should fight to bring together the margins of resection.
In case of the need of dealing with very large lozenge excisions or excisions located in convex regions of the body that normally generate higher tension forces with respect to flat areas, it is common the formation of “pockets” of exuberant skin and subcutaneous tissue taking triangular shape defined “dog ear.” Usually those pockets are located at the end of the suture, at the prongs of the breach in correspondence of the points in which the rotation axis converge, in case of rotation or transposition flaps, in the area of the mobilization for plain flaps. This tissue should be removed in order to obtain a linear suture (Fig. 22.2).
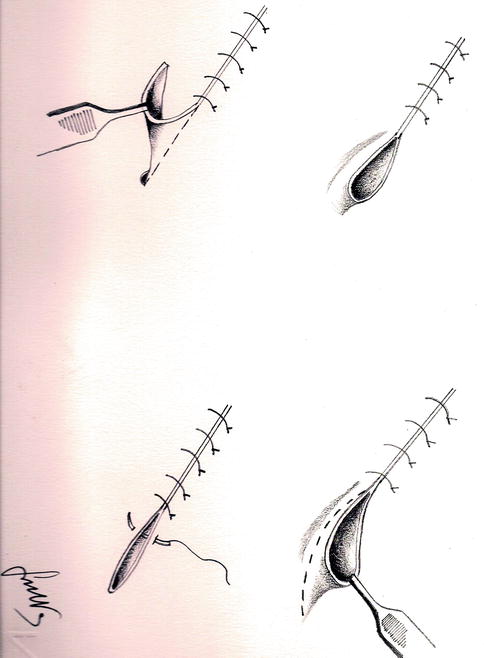

Fig. 22.2
“Dog ear” removal technique
The use of lozenge excision lends itself to numerous variations that can be adapted to the needs for size of the available skin, to the configuration of the lesion to be removed, etc. Still, the need to orient the major axis of the incision parallel to relaxed skin tension lines remains, changing the shape of the lines of incision in order to draw an “M,” a “Y,” a double “M,” a star, and other shapes (Fig. 22.3a–c).
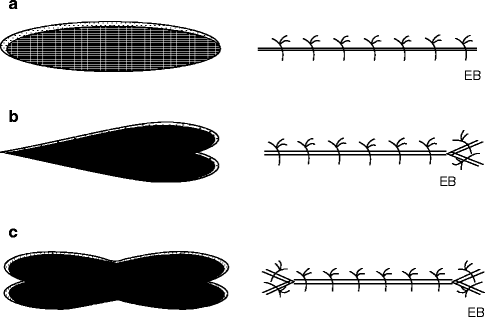

Fig. 22.3
(a–c) Incision line and shapes
Direct closure of the excision is not always possible and feasible because of excessive skin tension that would be generated, and that will increase the risk of diastase of the suture itself. In these cases, the surgeon will choose for an alternative solution such as seriate excision (multiple excisions spaced in time) or the use of skin expanders that create an tissue exuberance, following a slow but progressive tissue distension, which is necessary to cover the residual defect.
The use of grafts and/or flaps for the coverage of the surgical breach remains a valid alternative.
Seriate Excisions [25]
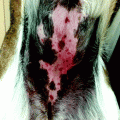
In general, seriate excisions are used to treat spindle-like damages or whose smaller diameter is in size too large to allow direct closure without the risk of diastase due to excessive tension generated, i.e., giant congenital nevus (Fig. 22.4).
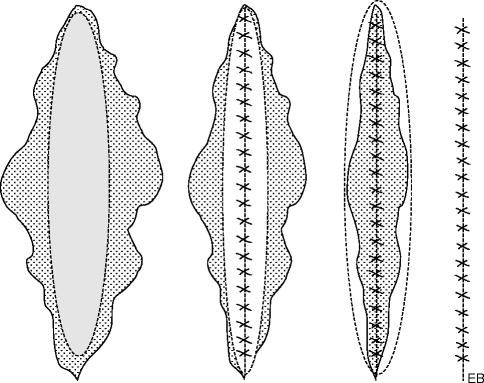

Fig. 22.4
Seriate excision
The technique involves at first an intralesional excision and in the end an extralesional one, through several sessions that should be at least 1 month apart. The procedure is to be repeated up to the point in which the complete removal of the skin formation is achieved. As mentioned before, the excision is carried out at the beginning without crossing the margins of the lesion that is to be surgically removed, using an incision shaped as a lozenge, as a double “M,” as an “M,” as a “Y,” etc. according to the most suited needs for shape, position, and extent of the breach.
Since this type of excision involves the development of a great amount of strain on the margins of suture, the surgeon must pay particular attention in obtaining a good matching of the skin edges of the wound, in order to not let them diastase until the next surgical session. As a result, the matching of the skin layers should be made through a careful approach of all the subcutaneous tissues, using absorbable suture, before skin closure. Especially during the last surgical round, the surgeon is required to pay accurate respect to aesthetics, having to remove during the entire process the scar tissue formed after the previous excision.
Hemostasis
Last of the surgical procedures to be performed during an operation, it still remains a procedure in surgery. Failure to comply with the accuracy in performing hemostasis involves unequivocally the formation of a hematoma in the context of the wound. This can delay or even prevent the physiological processes leading to the healing of the wound, resulting in a dehiscence of the suture and representing a perfect breeding ground for the development of pathogens and subsequent local or even systemic infection.
Careful hemostasis of small- and medium-diameter vessels can be accomplished in several ways: compression, plugging with H2O2, and suturing the vessels; the methodology that is more efficient, easier, and more rapidly put into practice is diathermocoagulation. This procedure uses the heat produced by the passage of electrical current through biological tissues. The procedure can be made more precise through the use of a bipolar forceps, which provides the current to flow only through the tips of the forceps, thus avoiding to cross the tissues that separate the positive from the negative pole. The bipolar forceps also allows the surgeon to limit the damage of burning the tissues surrounding the area of application of the current.
In the case where there are large caliber vessels or if it is not possible to apply electric current, for example, due to the presence of pacemakers, it is preferable to perform a ligation of these vessels with absorbable or nonabsorbable suture.
Whether doubts about the effectiveness of the hemostasis exist or in cases where bleeding may still be possible, as can occur in the face district, where vessels are subjected to an average blood pressure higher than other districts, the authors recommend the use of a drain to prevent the formation of a hematoma.
Medication
The quality of the scar, in cosmetic surgery, is considered a fundamental part of the action unlike other surgical specialties. It turns out to be one of the more visible consequences of the intervention. The authors are not asserting that when the surgery does not involve plastic surgery, the operator is permitted not to pay attention to the surgical suture of the breach; on the contrary, any surgeon should devote time and attention to the last note of an operation, which will be the one the patient will refer after surgery and after he or she had become sufficiently accustomed to the new body appearance.
Skin Expanders [25]
The use of skin expanders is now a viable alternative to seriate excision of large skin lesions. A skin expander is essentially a bag of silicone of various shapes and sizes, which is gradually filled through transcutaneous injections of sodium chloride solution. The filling is done through a valve integrated in the expander or positioned at a distance and connected through a tiny tube (Fig. 22.5). The valve is always placed subcutaneously and is palpable and detectable by means of magnets.
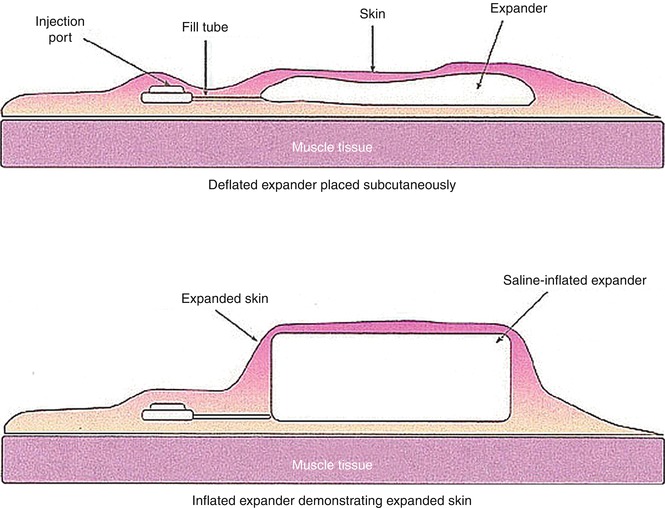

Fig. 22.5
Skin expander
The result of skin expansion is the formation exuberance of tissue, which can be used to surgically repair the breach. Of central importance is the identification of the area where the expander is placed, along with its shape and size. A placement in an location far apart from the excised area would result in difficulty using the obtained tissue, while, on the contrary, implant placement in an area immediately below the area to be excised would increase the amount of tissue excision. Incorrect positioning is also in the area immediately below the incision made for insertion of the expander, because it may lead to insufficient coverage or risk of rupture. Not to mention that incorrect placement may result in the presence of additional and unnecessary scarring.
To be evaluated when choosing to use skin expanders is the rate of the expansion, which must be adequate to avoid tissue damage from hypoperfusion, resulting in necrosis. The surgeon must remember that skin expansion necessarily involves pain in the affected area as a consequence of pressure. The period of expansion varies depending on the circumstances and the anatomical region but generally lasts a period of time ranging from 1 to 3 months.
As the sufficient skin expansion needed is reached, the expander is removed through a skin incision, while the fibrotic capsule that is formed as a result of skin reaction to the foreign body may be left in place.
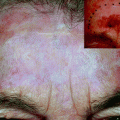
Skin expansion is indicated especially in the correction of extensive burn scars or posttraumatic removal of giant congenital nevi, in which skin needs to be expanded to reach a more acceptable cosmetic result. The main complications of this technique are represented by the ever-present possibility of infection, which will be necessarily followed by the removal of the expander, and the possibility of skin necrosis and pain during the expansion process. At any time the expander can undergo leaking, the result is the leakage of saline solution of NaCl in the same pocket where the expander is hosted, but makes it impossible to continue the expansion because the expander no longer is capable of exercise and maintain the necessary pressure. All this is associated with the disadvantage of a broad time necessary to achieve the required volume and defacement of body shape and appearance of the patient, especially if the expander is to be placed in exposed areas.
Recent scientific studies have established that the maximum percentage of tissue expansion obtainable never exceeds 130 %.
Practical Surgery
Although numerous techniques exist for the reconstruction of a tissue loss, it is possible to develop a scale for decision-making process that the surgeon should always keep in mind in working the choice of reconstructive method:


Whenever a direct closure is not possible, i.e., the suture of the margins of the wound, the surgeon would resort to use one of the following large sets:
1.
Grafts
2.
Flaps
According to the needs and complexity of the case (Fig. 22.6).
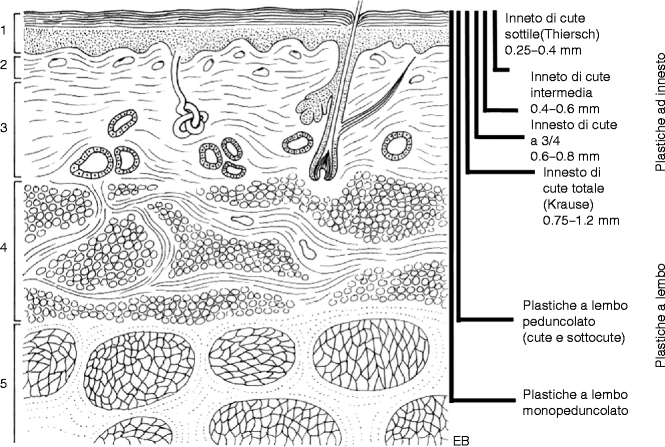

Fig. 22.6
Skin thickness versus graft and flaps
Grafts
A skin graft is a segment of one or more tissues that have lost all connection with the donor area, does not contain a network of blood vessels of its own, and can be transferred from a donor site to a receiving area of substance loss (deficit), for reconstructive purposes, resulting from trauma or tumor excision.
There is considerable confusion about the classification and definition of key elements such as grafts, plasty, and flaps. Many authors use the term “skin graft” to refer to the transfer of tissue from a donor site to the recipient site, assuming the only differentiation into two main groups: grafts and flaps. For those authors the term grafting involves transplanting a portion of skin variable in thickness and other measurements which, totally separate from the donor seat, is capable of fully nourishing itself at the expense of the new seat, unlike the flap that retains a vascular link with the origin site [26].
On the contrary, other authors (e.g. Kirschner) consider part of the definition of “flap” the whole part, either referring to open and/or closed (tube) flaps or to simple grafts. There even are authors who use the term “local grafts” to indicate local skin plasty (sliding, rotational flaps, etc.) [27].
The differences are significant and cause a confusion of terms and ideas, if some simple basic concepts are defined and can be enough to thin out the haze: the authors consider a skin graft including all the skin grafted which is required to survive entirely in the new site. The definition does not exclude the vessel disconnection from the donor site to take place in a less abrupt and more gradual way, allowing the new site to ensure the survival of the transplanted tissue. Exactly this is the difference between free and pedicled graft, and it is on this difference that the classification of direct engagement (or free) and mediated (or pedunculated) flap relies. Besides, the term flap may not be comparable to graft, since the latter is something concrete and definite (with its own personality and character), while the former is a surgical technique used to make plasty correction by means of local transfer of neighboring tissues (feed, slip), pedicled or remote (microsurgery) flaps [28].
Reverdin in 1869 presented a paper that refers to the method of free transfer of small segments of epidermis. Since then, the number of classifications and names proposed for this surgical technique, adopted by authors from different schools, was so large, diverse, and sometimes contradictory that it would be essentially impossible to make a really complete list. From a strictly anatomical point of view, it is possible to distinguish grafts as epidermal, dermoepidermal, dermal, and full thickness, depending on the depth. however, if, we consider the evidence that the latter (epidermal) are not feasible in practice, because it is impossible to get them from any donor area, with the exception of those taken at the plantar region as demonstrated by J.S. Davis that studied seriate sections of the skin, that there is an obstacle distinguishing the different layers, identifying the real plane of dissection of the graft itself, in this chapter the authors propose to simply adopt the anatomical classification. To demonstrate the desirability of this classification, we can mention a few emblematic examples from the best known classifications: Thiersch grafts (the thinner dermoepidermal) are called “split” grafts, fine section grafts (Blair Brown), epidermal grafts (Barsty), fine grafts extracted with razor (Gillies), Ollier-Thiersch surface grafts (Sanchez Arbid), etc.; dermoepidermal grafts of intermediate thickness are called “split” grafts (Blair Brown), roughly split graft (Harkins), large grafts extracted with razor (Gillies), Ollier-Thiersch deep grafts (Sanchez Arbid), intermediate depth grafts (Padgett), etc.; and dermoepidermal large grafts are called calibrated grafts (Harkins), grafts intermediate depth (Padget), big “split” grafts (Blair Brown), etc. In addition, Kirschner believes that while the classic Reverdin graft includes all the layers of the skin, the majority of authors use the name of Reverdin grafts for small dermoepidermal superficial grafts and there are those who distinguish the two types (superficial and deep) as Reverdin graft (Sanchez Arbid) [6].
Island Grafts [25]
The present array includes two different major groups:
1.
Reverdin, or superficial, which includes the epidermis and upper layers of the papillary dermis
2.
Davis or deep, described by S.J. Davis in 1914, embracing all the skin layers (epidermis, papillary dermis, corium, and hypodermis) in the shape of an inverted cone
Among the variants of the island grafts, we can consider the so-called Wagenstein “sprouts” grafts, which in truth are nothing more than the Reverdin-type grafts placed deeply in the granulation tissue.
Laminar Grafts [25]
They match, as we said before, the “split” grafts of Anglo-Saxon source and include all large dermoepidermal grafts regardless of thickness, but do not include all skin layers. There are three types of laminar grafts:
1.
Thiersch (or thin), described in 1874, which is equivalent to the laminar Reverdin island graft, one third of the total skin (from 0.2 to 0.25 mm) depth, containing part of the epidermis and papillary dermis.
2.
Blair Brown (of intermediate thickness), described in 1929, is about half the thickness of the skin in depth (which includes a thickness selected among 1/3 and 2/3 of the total skin section), whose size varies from 0.3 to 0.4 mm. It is the most widely used to treat early deep burns.
3.
Padgett (or thick), subject to precise specified measures by the author techniques in 1939 (Harkins calibrated grafts), otherwise known as “trocar graft” because it includes more than 2/3 of the skin section, it is harvested at about 0.5–0.6 mm deep and includes the epidermis, the papillary dermis, and most of the deep dermis or corium.
4.
Brown “implant” grafts and Goode-Gabarra “matrix” grafts are technical variations to the application of either type of laminar graft mentioned.
Total Skin Grafts [14, 25]
They are made including all the skin layers. Used by Wolfe in 1875 to reconstruct the lower eyelid, they were introduced and described in detail by Fedor Krause in 1893. Although the success of the application is tied to a lot of attention, needed more than for other types of grafting, the aesthetic and functional results are much higher than those of any other type of free skin graft (because the morphology and physiology are exactly the same as those of normal skin). The Douglas grafts or “sieve” like and those “tunnel” like or Keller are Wolfer-Krause grafts, with slight technical variations.
Dermal Grafts [14, 25]
Introduced by Loewe in 1913 for the treatment of hernias and reinforcement of the abdominal wall, dermal grafts find multiple indications in surgical practice, successfully used for the repair of tendons, meninx, ligaments, etc. and especially as autologous material for sutures. Cannady deems dermal grafts as the autoplastic material of choice, superior even to the fascia lata. Plastic surgery is currently dominated by two applications of the dermal grafts:
As filler for the correction of craniofacial defects (Eitner, 1920).
To repair skin losses just like dermoepidermal grafts, through the lamination process (Zintel technique) of a thick Padgett laminar graft, previously obtained with the dermatome, in this way it can be completely divided through its cross section so as to double the surface of the obtained graft. The dermal graft obtained in this way is suitable to cover the receiving site surface and plays to perfection the task of repairing the skin like any other laminar graft. The application of these dermal grafts is extremely useful in the treatment of extensive burns, where donor areas are reduced in number and extension. It therefore opens new possibilities thanks to the “laminated grafts,” a name that indicates the origin and clearly distinguishes them from the laminar grafts (dermoepidermal) to avoid possible confusion in interpretation and nomenclature.
Pedicle or Mediated Grafts [29]
Include all those skin grafts or transplants that are temporarily maintaining vascular connections with the donor area, up to the time in which nutrition of the tissue transplanted is actually at the expense of the new site. Mediated or pedicled grafts can be applied directly, where the dissected flap is applied directly on the area to repair, or indirectly, where the transfer of tissue takes place by successive steps, through an area or region that is intermediate (temporary host). Either direct or indirect mediated flaps can be prepared in an open or closed shape, depending on whether or not they are isolated from the outside of the cruented side. The “closure” of a flap can be done through three different procedures:
The process of tubular flaps by Gillies-Filatov
Covering the cruented surface of the flap by applying a laminar graft
Through the juxtaposition of another similar pedicle flap but of opposite base, taken from the receiving or intermediate area depending on whether it is a direct or indirect pedicle graft.
Direct Pedicled Grafts
Include grafts made directly from the donor site to the one intended for repair. They can be distinguished as follows:
Near flap, taken from regions adjacent or close to the defect. They are used mainly for the repair of craniofacial loss of substance; flaps can be based on a single vessel (e.g., the classic Indian flap nasal reconstruction based on a front flap) or double-pedicle flaps, such as the flap called “a helmet,” mainly used to repair areas of the chin at the expense of the hairy scalp;
Direct remote flaps, where the transplant takes place between regions anatomically distant from each other but can be approached and kept in this last position (e.g., using a cast) for the time necessary to make the flap root in the new site; these types of flaps include the so-called direct distance flap crossing arms and legs of the patient (“cross-arm flaps” and “cross-leg flap” according to the Anglo-Saxon authors), used to repair the upper and lower limbs.
Indirect Pedicled Grafts
They are made via an intermediate zone or “temporary” host. It is worth quoting “larva” flaps or “seriate jumps” flaps Hahn’s “migrant” flaps, in which the approach to the skin defect is done by means of subsequent steps, alternatively dissecting either one of the pedicles of the bridge that forms the tubular flap and then replanting it each time closer to the receiving site.
among indirect pedicle grafts, the transported flaps (with intermediate Schrody’s carrier), in which the transplant is done at the expense of a host area. The flap can be rolled in a tubular shape, usually using the wrist as the intermediate zone, or in the form of wide transported flap (the Anglo-Saxon “closed carried flap”) with the juxtaposition of two very broad-based pedicle flaps in the abdomen and in the forearm. The abdominal flap will be placed permanently in the area to be repaired, while the forearm (which served as a nutrient base to another provisional) will be retrieved to the original site.
The skin graft is a graft that must live at the total expense of the new recipient area. It is defined as free graft (or immediate) when the nutritional gap is abrupt, occurring in a single operative time, and it is called pedicle graft (or mediated) if the transfer takes place keeping those connections that ensure temporary nutrition through the vascular pedicle which joins the donor area, until it has completed the formation of the vascular network that ensures the survival of the graft at the expense of the new site.
A simplified classification is based on grafts’ [14, 17, 25]:
1.
Antigenicity
2.
Seat
3.
Composition
1.
Antigenicity (increasing)
(a)
Autologous: Donor and recipient are the same individual.
(b)
Homologous: Donor and recipient are different individuals but belong to the same species. In the event that it presents the same antigenic structure, such as identical twins, it is defined as “isografts”; otherwise, it is defined as “allografts.”
(c)
Heterologous: Donor and recipient belong to different species.
2.
Location
(a)
Isotopic: tissue transplants with identical characteristics to the tissue of the receiving area
(b)
Heterotopic: tissue transplants with different characteristics from those of the recipient
3.
Histological composition
(a)
Simple: consisting of a single tissue
(b)
Compound: made up of several tissues
For simplicity and quickness of execution and the multiplicity of applications, skin grafts are widely used and have broad indication in surgical practice today. The class of grafts which are used most frequently is that of autologous grafts, in which the individual donor and recipient match. Even because a proper packaging and placement are rarely followed by complications, still easy to manage. On the other hand, the use of homologous and heterologous grafting is now largely reduced compared to the past, because of the strong immunogenicity which results in significant compatibility issues, which are followed by complete digestion and subsequent rejection of the transplanted tissue. Their main indication is the management of large burned as a biological dressing.
Special surgical instruments called dermatome, manually or electrically operated and adjustable in thickness, are used for the harvesting of the desired graft. The use of electric dermatome is usually associated with a higher precision in thickness and greater uniformity with respect to harvesting made with a hand dermatome.
Autologous skin grafts (or autografts) can be further divided into the following:
1.
Partial-thickness skin grafts
2.
Full-thickness skin grafts
Partial-Thickness Grafts [14, 17, 25]
The partial-thickness grafts are mostly used as a cover in losses of substance of wide portion of the skin surface. Their thickness includes the epidermis and the dermis, even if the total thickness varies according to the usage meant, being differentiable as partial-thickness thin, average, and total skin grafts.
This set of grafts is characterized by a much faster revascularization with respect to the one that includes full-thickness grafts, but more frequently presents complications such as higher percentage of retraction, higher frequency of color alteration in the sense of hyper- or hypopigmentation, and poor coverage of deeper tissues. The shrinkage occurs mainly after engraftment and is due to the physiological contraction of scar tissue that is interposed between the graft and the recipient site (secondary retraction). This phenomenon, which can last several months, may result in the reduction of the surface of the grafts up to 50 % of the total. The color alteration that may occur as a complication of partial-thickness graft is a consequence of the action of hormones and ultraviolet light on the melanophores present in the grafted dermis.
The donor area for a partial-thickness graft heals by second intention; in other words, reepithelialization starts from the remaining skin appendages, with almost complete restitutio ad integrum. As logic dictates, the thinner the graft, the greater the amount of adnexal residual are left in the donor area and much quicker the donor site heals: in the case of a thin graft, healing time is about 7–9 days, while if the same area undergoes intermediate-thickness harvesting, it will heal within 10–14 days; finally, if it is harvested, a full-thickness graft reepithelialization time can stretch to nearly 3 weeks. The extension of the healing time is due to the gradual reduction of adnexal residual, which is directly proportional to the thickness of the graft. Healing will progress forming granulations in the area of the harvesting beginning from the edges of the wound, with a slower process and a greater probability of hypertrophic scars formation. In this case, average and full-thickness harvests must then be covered with a thin graft.
To increase the surface of the skin grafts, thin or full-thickness, an instrument called a Mesher is used: the procedure involves the passage of the graft through the rolls of the instruments that are capable of cutting full thickness of the sheet of skin in a regularly seriate cutting, changing the solid layer into a net (mesh graft) which will heal starting from the edges of the mesh. The size of the net is adjustable, changing the expansion ratio as surgery demands. The mesh graft has the advantage of allowing the coverage of extensive loss of substance even when they have limited amount of tissue harvested; thus, the presence of fenestrations (mesh) promotes the drainage of any collected blood and serous, reducing complications such as hematoma and seromas.
Full-Thickness Grafts [14, 17, 25]
A full-thickness graft is a graft in which the harvesting is deepened to include the whole skin, i.e., epidermis and dermis. This harvesting is performed with a scalpel and not with the dermatome; any remaining fat residuals of the subcutaneous tissue is carefully removed using sharp instruments, such as scissors, because it may prevent implantation of the graft itself. As a consequence of the important thickness of the graft, the timing of revascularization will be longer than any other thinner graft. Precisely, it is the thickness that reduces the frequency of complications such as retraction, which in this case is primary; in fact it is the result of the presence of elastic fibers into the skin, which will contract immediately after harvesting. Even color alteration, greater resistance, and coverage of deep layers are consequences of the greater thickness. Unlike partial-thickness graft, the donor area needs to be sutured, as of any subsequent breach of a surgical excision, to approach the edges. This detail limits the harvesting areas for full- thickness grafts to those in which the tissue elasticity allows a good distension of the skin, it allows to efficiently conceal the residual scar and limits the extent of the possible harvesting. The main indication for use of full-thickness grafts is for limited coverage areas, small and medium sized, and whose bed is normo-vascularized. As with any thickness grafts, it is necessary that infection is not present in the recipient area. The end result is usually aesthetically more desirable with respect to a partial-thickness graft.
Virtually the entire body surface can be considered as a donor area for partial-thickness grafts; much more limited appears to be the body surface that can serve as a donor area for full-thickness grafts. The face is still excluded from this count. Common sense has it that the search of the donor site should begin in the immediate vicinity of the recipient site, in order to ensure an effective residual scar concealment, and only subsequently other donor sites will be considered for harvesting such as the one on the medial thighs, arms, or buttocks. In case of a need for full-thickness graft, areas that can be taken into consideration with the before-mentioned requirements are far less extensive than in previous and are limited to the retroauricular region, the inguinal fold, the base of the neck, and the lower fold of the buttock. The rule stands as in preferring harvesting in areas where the tissue is most similar to the one present around the receiving site, for a more acceptable cosmetic result.
The complications associated with skin graft surgery are substantially comparable to those of any other surgical procedure; these must be added to those that we can define as “proper” of the surgical method in question and which are represented by the possibility of shrinkage and alteration of the skin color, which, respectively, are directly and inversely proportional to the thickness of the skin graft.
Surgical Technique [30]
To allow revascularization of a graft, exact conditions must be met. The graft must have a regular thickness and be totally free from residues of the subcutaneous fat, and the recipient site must be cleaned, free of necrotic debris and foreign bodies, and well vascularized to allow for immediate survival. The contact between graft and recipient area must be secured by a moderate pressure and a strict immobilization.
The harvesting of a skin graft is performed with a scalpel when a full-thickness graft is needed and in other cases using special devices called dermatomes (Fig. 22.7).
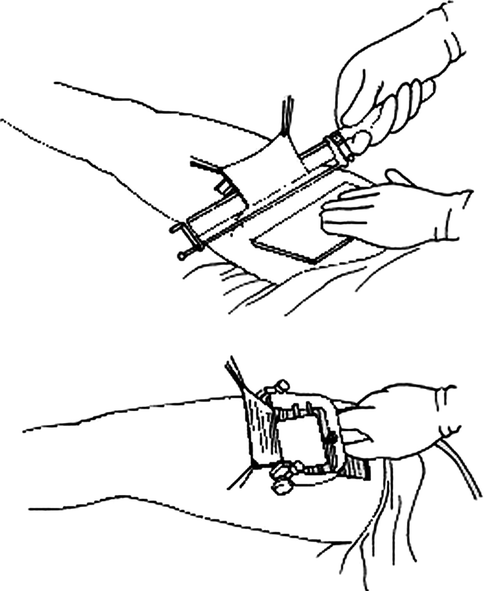

Fig. 22.7
Dermatome
Several types exist:
Adjustable blade knife: It is quick and easy to use but requires good manual experience.
Drum dermatome: It is of a more complex use but allows very precise and regular graft harvesting, nowadays fell into disuse.
Electric and pneumatic dermatome: Easier to control; they can be used even by inexperienced surgeons and in harvesting in areas with uneven surface.
The areas generally considered more suitable for harvesting a graft of thin or medium thickness are the thighs, the buttocks, and the abdomen, being large, relatively flat areas, allowing large skin harvesting. In these areas, scars are also easily concealed beneath clothing, thus reducing the patient’s discomfort for possible cosmetic damage. For the harvesting of a full-thickness skin graft, preferred areas are with soft, thick, and colorful evenness, such as the retroauricular region, supraclavicular region, the elbow fold, the groin, and the inner surface of the arm.
The healing period of the donor area varies depending on the thickness of the graft harvested. Sure enough, in the case of skin harvesting of thin or average thickness, the donor area tends to heal spontaneously by reepithelialization, beginning from the bottom of the bed of the wound and will include the skin adnexal. The healing will be complete within 15–21 days. After the time interval, if necessary, harvesting in the same area can be repeated.
In the case of harvesting three quarters of or full-thickness graft, spontaneous healing can occur only by secondary intention. Margins should be approached if the donor is narrow; when surfaces are larger, a thin graft harvested from an adjacent area can be used to cover the first donor site. The repair by secondary intention leads to a rather slow recovery, with formation of scars in general exuberant and hyperpigmented.
Mode of Engraftment [30]
Graft harvesting disconnects all vascular and nervous connections with the donor area, and the appearance of the tissue immediately after is intensely pale. The contact between graft and recipient area is initially established through a weave of fibrin, while the survival of the graft cells, during the period of time elapsed from the harvesting moment to the complete recovery of vascular connections, is ensured by the fluid exuding in the recipient region. This process, previously mistakenly referred to as plasma circulation, is today more properly defined as serum imbibition and is capable of providing a valuable contribution for the nutrition to grafted tissues throughout a 2-day time period. Relatively recent scientific studies have led us to believe that the role of the vascular network, the grafted tissues, is only transitory and that these vessels act as a guide for the reorganization of the local vascular network. Sure enough, only 5 h after surgery, in the bed of the receiving area, several endothelial mitoses can be observed beginning from the bottom and the margins of the wound, finally establishing vascular connections to the recipient site vessels. The first vascular anastomoses between the graft and the host area are documentable 24–72 h after surgery. A real blood flow in the graft is evident already starting on the third day after surgery, and revascularization is completed between the sixth and the seventh day, with the differentiation between arterioles and venules which is completed on the eighth day. The blood flow in the graft stabilized itself to normal within 20 days from surgery. These statistics give us the reason why it is strictly necessary to immobilize the graft during the first 5–7 days post-surgery and the problems thicker grafts have to take root, considering the more complex skin structure and the increased effort needed to complete the formation of the vascular network. Within 3 months after the operation, reinnervations is completed but is often accompanied by subjective disorders such as paresthesia and hyperesthesia with spatial dissociation. With regard to skin adnexal, the sweating function is kept limitedly to a slight recovery only in the full-thickness grafts, while sebum-secreting function may also be present in the thinner grafts.
The graft revascularization follows three different mechanisms:
1.
Inoculation: Direct reconnection of the vessels of the graft to the vessels of the recipient area.
2.
Vessel growth: Host vessels grow to from anastomosis with the graft vessels.
3.
New formation: A new vascular network is formed beginning from the host vessels.
Flaps [26]
A flap is defined as a transfer of one or more tissues from a donor site to a receiving site while maintaining integrity in the vascular network. same as, transferring vascular connections to supply the tissue. This blood supply can be maintained in connection with the original one, as in the case of local tissue flaps, or reconnected to the vascular network of the recipient site, as in the case of the distance flaps. As with any surgery, the planning phase is of fundamental importance for the indication, the study of the vascular anatomy of the areas, and the preoperative drawings.
Flaps can be classified considering the following:
Anatomical relationships | Local | Sliding |
Rotational | ||
Transpositional | ||
Z, M, trident, W, etc. | ||
Distant | Direct | |
Tubular | ||
Free or microvascular | ||
Composition | Simple | Cutaneous |
Fascial | ||
Muscular | ||
Osseous | ||
Composite | Fasciocutaneous | |
Myocutaneous | ||
Osteocutaneous | ||
Adipose-fascial | ||
Myofascial | ||
Vascularization | Axial | Direct flow |
Reverse flow | ||
Random |
Local Flaps
The indication of local flaps is the closure of defects adjacent to the donor site. To get the best aesthetic and functional results in the coverage of a defect is recommended the use of the skin adjacent of the defect itself. These flaps are composed of skin and subcutaneous tissue, and their transfer to cover the surgical breach is carried out through surgical procedures that can be classified as sliding, rotation, and transposition. Obviously, the procedure to be adopted is related to the size of excision, the anatomical site in which the excision occurs, and the orientation with respect to the relaxed skin tension lines, in other words, to the skin tension that is generated as a result of excision.
As the general surgical approach should be to make the excision with the specific intention of direct closing the wound suturing, not always easy, likewise when choosing for the use of flaps, the surgeon should aim for direct closure of the secondary defect. Surgical practice has pointed out that even this approach will not always be easy; in these cases it would be necessary to close the secondary defect by means of skin grafting to preserve the underlying tissues and anatomical structures. Of central importance is the accurate per-operatory planning, dedicating time to the careful measurement and delimitation of excision areas, to evaluating the size and scope of harvesting and to the study of relaxed skin tension lines as they appear before and after the flaps are transferred, as well as to the perfusion topography of the areas involved. The same careful attention must be paid in respecting local conditions existing in the area before surgery. As for the lozenge excisions, the mobilization of margins is followed by a reduction of the tension exerting on them, as well, in a reduction of the most frequent complication that might happen: necrosis. Sometimes it will be necessary and appropriate to leave in place a fall drainage, which must be maintained during the first 2–3 postoperative days. The medication applied should always be moderately compressive, in order to avoid hypoperfusion for the vascular pedicle of the flap from excessive compression.
Sliding Flaps
The result of excision and removal of skin area is the formation of a breach in the outer envelope of the human body. The repair of the breach usually is carried out with direct suturing, even after loosening the margins of the breach itself and stretching of the adjacent skin. The tension exerted to dislodge the mobilized skin will be directly exerted on the margins of the wound itself and precisely where the suture maintains those margins adjacent. If the traction is excessive, it may expose the suture to risk of diastases; the tension can be dissipated engraving one or more “V” incisions, both superiorly and inferiorly. The suture is performed so to change the “V” shape to a “Y” shape, “V-Y” plasty, increasing the mobilization of the tissue of the area, thereby reducing the tension on the margins of the wound. This simple method can be applied whenever it is necessary to unload the excessive traction as can result, for example, from plastic elongation of retracting scars.
Today, flap surgery offers more than proven solutions that supply remarkable plasticity and adaptability to surgical needs. The patterns of etching techniques are shown in Fig. 22.8.
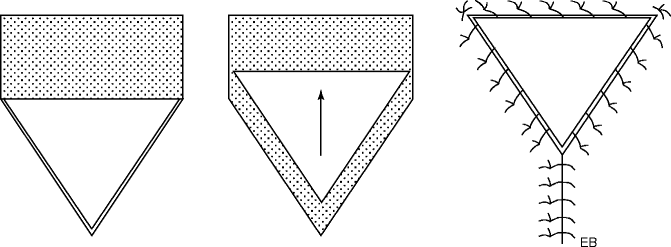

Fig. 22.8
“V-Y” sliding flap
Advancement Flaps
The transfer of this flap type occurs following a linear path from the donor to the receiving site, along its longitudinal axis. The shape varies considerably depending on the defect to be repaired and the anatomical region; even if most often are rectangular in shape, they can be shaped as V’s, U’s, and H’s, usually with a length/width ratio of 2.5–3:1 cm. Close to the proximal end of the flap, a triangular incision should be made to exceed the exuberant tissue present. The indication for the use of this set of flaps is the repair of a small skin defect, up to a maximum of 2 cm in length (Fig. 22.9).
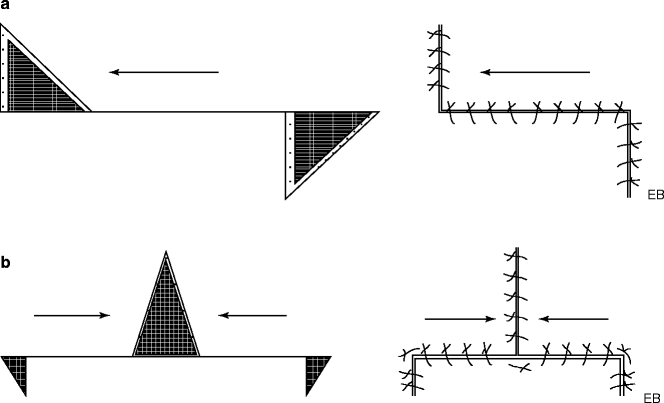

Fig. 22.9
Advancement flaps. (a) Lateral advancement. (b) Bilateral advancement
Rotational Flaps
Similar to the previous set of flaps for what concerns the surgical technique, it differs for the incisions, distinguished by having a semicircular or arched shape, which rotate along an arc in the direction of the defect until the skin defect is completely covered. Also in this case, to reduce the tension mounting in the area, an incision can be made or a triangle of skin can be removed near the base of the flap (Burow’s triangle). Depending on the needs, flaps can be of various shapes but retaining the intrinsic property to rotate sideways. The donor site is closed either through redistribution of tissue and direct suture of the breach or by skin grafting if the residual defect is particularly large or is located in body areas where the tissues are adherent to the underlying bony structures such as the scalp.
Transposition Flaps
A transposition flap is defined as a flap in which the tissues used to repair the defect “leap over healthy tissue,” turning and covering all or part of the primary defect. These flaps are of different shapes, arcuate, angular, sharp, “V” shaped, “W” shaped, “trident” shaped, and “Z” shaped, and are generally carved at one edge of the defect. The donor site is then closed by direct suture, skin graft, or a secondary flap, such as the bilobed flap.
Peculiar and widely used is the Limberg flap, described for the first time in 1946. It is a diamond-shaped transposition flap. The area to be excised is inscribed in a rhomb with angles of 60° and 120°, planned in close relation to the amount of tissue available to cover the resulting defect. The uniqueness comes from the ability to choose one of the four sides of the diamond that will serve as the pedicle of the flap. When transportation direction is chosen, the incisions will be oriented according to the relaxed skin tension lines, prolonging the minor diagonal throughout its length. At the end of the extension, a line parallel to the side of the diamond and of equal length is incised. The outcome is a flap whose three sides and whose bases are of equal length, whose corners coincide with those of the breach. After the transposition, the donor site is sutured directly. The association of more than one Limberg flap is possible if the skin defect is of wide proportions (Fig. 22.10).
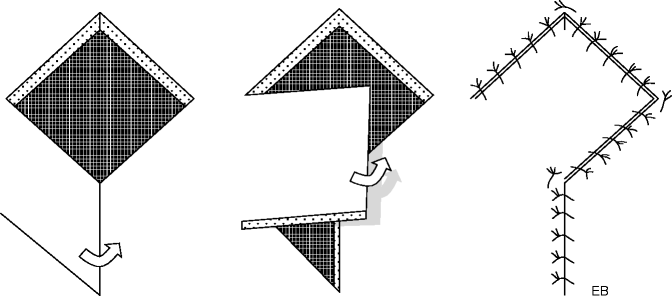

Fig. 22.10
Transposition flaps
Variant of the previous flap is the flap of Dufourmentel, described for the first time in 1962. In this case the defect is inscribed into a rhomb, whose angles are 30° and 150°. The first side is drawn along the bisector of the angle opposite to the one formed by one of the sides and the minor diagonal, and the second side is parallel to the major diagonal and of equal length. The advantages over a Limberg flap are the following: less tension acting on the suture of the donor site and greater availability of the tissue to cover the defect (Fig. 22.11).
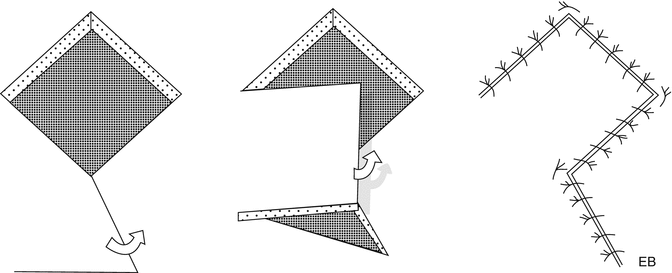

Fig. 22.11
Dufourmentel transposition flap
Differently addressed is the “Z” plasty, which is useful in stretching a surgical incision or to reorient the direction of a scar, resulting in lengthening of the tissues present, in the redistribution of tension exerted over the surrounding areas and the disruption of the major axis of the scar. The technique involves the exchange of opposing skin flaps, the side incisions will be long as the lateral axis, and the angle of incision can have values between 30° and 60° with respect to the direction of the scar, remembering that a smaller angle increases the risk of necrosis of the tip of the flap, while any larger angle makes it difficult to rotate the flap itself. The length of the central axis determines the value of the final gain, while the amplitude of the corners determines the amount of lengthening achieved. With 30° angle, the resulting increase in the length would be around 25 %; with angle of 45°, the percentage rises to 50 %; and with amplitudes of 60°, resulting gain reaches of about 75 %. From practical experience, the authors have noted that the amplitude of the most useful angle is 60°, because more acute angles determine insufficient elongation, while more obtuse angles result in an excessive increase in the tension of the adjacent tissues, making it difficult to transpose the flaps. This kind of technique can be applied as seriate surgery, multiple Z plasty, if the amount of skin available is not sufficient or in case of injury of large dimensions. The “Z” variant is used primarily in flexor regions (Fig. 22.12).
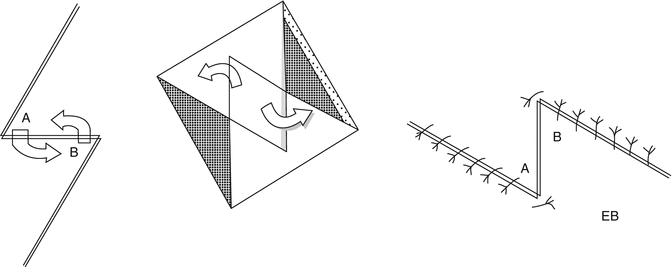

Fig. 22.12
“Z” plasty flaps. A: superior flap. B: inferior flap
Distant Flaps [26]
When near the repair area is not possible to find enough tissues, flaps must be moved from distant donor sites. This can be achieved with the flap temporarily joining two nonanatomically contiguous areas. The transplant performed can be separated from the donor site only after a period of at least 3 weeks, because it needs to establish vascular connections to the vascular network of the recipient site, so to allow disconnection of the pedicle without damaging the viability of the flap. This method has, compared to proximity flap, some negative aspects for the two anatomical regions, joined by the transplant, must be kept in strict immobility and the posture is often not comfortable for patients. In particular in elderly patients, it may result in joint ankylosis not easily solvable. Thus, being necessary more than one operation, the patient is exposed even to risks deriving from repeated anesthesia and contamination, along with the problem of prolonged hospitalization.
Depending on the anatomical regions involved, different types of flaps are characterized:
Direct
Tubular
Free or microvascular
Direct Flaps
The term “cross leg” refers to the repair of a loss of substance of a lower limb using a direct, distance flap from the contralateral limb; the flap is usually obtained from the sural region or from the thigh. The indication for this flap is the repair of lesions with exposed tendons and bones, osteomyelitis, skin fistulas, or necrosis deriving from exposed fractures with metal plates and screws system.
“Cross arm”: The flap is drawn on the inner surface of the arm and used to repair injuries of the contralateral limb, in particular of the hand.
“Cross finger”: Repair the volar surface of a finger using a strip obtained from the interdigital surface of an adjacent finger. To repair losses of substance of the fingertips, a similar flap harvested from the thenar region can be used [31].
A historical example of a flap harvested from the inner face of the arm used to repair a loss of substance of the face, in particular the nose, is known as the Italian method. The thorax and abdomen can also provide plenty of skin for the repair of losses of substance of the upper limbs, which are easily approached and kept in contact with them.
Tubular Flaps
Nowadays rarely used, a tubular flap is bipedicle, plan flap, sutured to itself along the two long sides to form a canal with on the outer surface the skin and subcutaneous part on the inside. The flap can have a considerable length because it is sufficiently fed by the two pedicles.
Its main indication is the need to transfer a lot of skin at a great distance.
The tubular flap is harvested in preferential areas such as the base of the anterior cervical region, the abdomen, the pectoral region, and the inside face of the arm. After 3 weeks of its harvesting, the transfer can take place; it can be direct if the donor and the receiving site are close or can be brought together or indirect through a first transfer to a carrier region, normally represented by the wrist. Once the flap has reached, its final location is unfolded along the ventral scar and set in place.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree