Pilar tumours
Pilar adenoma immunoprofile is similar to basal-cell carcinoma (BCC) of skin: epithelial membrane antigen (EMA), carcinoembryonic antigen, S100, CD15 and CA72.4 are negative
Desmoplastic trichoepithelioma versus infiltrative BCC: EMA, CD15, chromogranin, CK20; these markers are largely negative in invasive BCC
IBCC stromal cells are stromelysin-3+
Table 6.2
Immunohistochemical features of sebaceous tumours
Sebaceous tumours |
|---|
Absent expression of S-100, CA72.4, GCDFP-15, CEA compared with sweat gland tumours, which are positive for these markers |
Sebaceous and sweat gland tumours CD15+ and BerEP4+ |
EMA characteristic ‘bubbly’ cytoplasmic decoration |
Table 6.3
Immunohistochemical features of sweat gland tumours
Sweat gland tumours |
|---|
Express CK, CEA, CA72.4, CD15 and p63 |
Epithelial membrane antigen more common in malignant neoplasms |
Eccrine carcinomas S-100+, apocrine S-100−, GCDFP-15+ |
Paget’s disease: CK7+, GCDFP-15+, BerEP4+, CA72.4+ |
Malignant melanoma: negative for Paget’s disease markers, HMB-45+, melan-A+, S-100+ |
Bowen disease: AE1/AE3 and K903+, negative for Paget’s disease and melanoma markers |
The diagnosis of malignancy often offers difficulties to the pathologist. Generally benign lesions are multilobulated and symmetric with smooth borders. Thus, tumour cells are almost monomorphous and no necrosis is observed. Malignant features include asymmetrical growth, infiltrative borders, irregular arrangement of the neoplastic cells, cytonuclear atypia and increased mitotic activity. Tumour necrosis and superficial ulcerations are commonly seen. The patients should be closely followed up for potential regional and distant metastasis.
Pilosebaceous Tumours
Histology of Pilosebaceous Apparatus
Skin appendages derives from ectoderm and are localised in the superficial and deep dermis and in the epidermal tissue. Classically they are divided into three distinct structures: (1) the pilosebaceous unit, (2) the eccrine sweat glands and (3) the apocrine glands. The distribution of these structures varies dependently from skin anatomic area. The pilosebaceous units are constituted by hair follicle and connected sebaceous glands. The hair follicle develops from the epidermis, as a deep invagination, and it forms the hair. The lower portion of hair follicle is located in the reticular dermis and subcutaneous fat and is constituted by connective papilla enclosed in the hair bulb. The cells lining the dermal papilla are involved in forming the hair shaft and internal root sheath. All sebaceous glands are associated to follicle, with the exception of the labia minor and glans penis. They are lobular glands, constituted by sebocytes, i.e. cells containing lipid vacuoles and lined by a single layer of dark, germinative cells. The product of sebocytes converges in short duct till hair follicle [6].
Pilosebaceous Tumours Classification
Among pilosebaceous proliferations, three categories have been considered: hamartomatous proliferation, benign tumours and malignant tumours. In each one of the categories, sebaceous and pilar derivations have to be recorded (Table 6.4).
Table 6.4
Pilosebaceous tumours distribution
Hyperplastic and hamartomatous lesions | Benign neoplasms | Malignant neoplasms | |||
|---|---|---|---|---|---|
Hair and hair follicle | Sebaceous glands | Hair and hair follicle | Sebaceous glands | Hair and hair follicle | Sebaceous glands |
Basaloid follicular hamartoma | Sebaceous hyperplasia | Trichofolliculoma | Sebaceous adenoma | Trichilemmal carcinoma | Sebaceous carcinoma |
Basaloid epidermal proliferation | Nevus sebaceous of Jodassohn | Desmoplastic trichoepithelioma | Sebaceoma/sebaceous epithelioma | Trichoblastic carcinoma | Basal-cell carcinoma with sebaceous differentiation |
Overlying dermal mesenchymal lesions | Trichoblastoma | Malignant proliferating trichilemmal cyst | |||
Trichofolliculoma | Trichoblastic fibroma | Pilomatrix carcinoma | |||
Sebaceous trichofolliculoma | Trichoadenoma | ||||
Folliculosebaceous cystic hamartoma | Proliferating trichilemmal cyst/pilar tumour | ||||
Trichodiscoma/fibrofolliculoma | Trichilemmoma | ||||
Pilar sheath acanthoma | Desmoplastic trichilemmoma | ||||
Pilomatricoma/proliferative pilomatricoma | |||||
Hamartomatous Proliferation
Hamartomatous proliferations recognise different clinic-pathological often unrelated lesions of (1) pilar origin (basaloid follicular hamartoma, basaloid epidermal proliferation and the group of trichofolliculoma–like lesions, including trichofolliculoma, sebaceous trichofolliculoma, folliculosebaceous cystic hamartoma, trichodiscoma/fibrofolliculoma and pilar sheath acanthoma) and of (2) sebaceous origin (sebaceous hyperplasia and nevus sebaceous of Jadassohn) (Fig. 6.1).
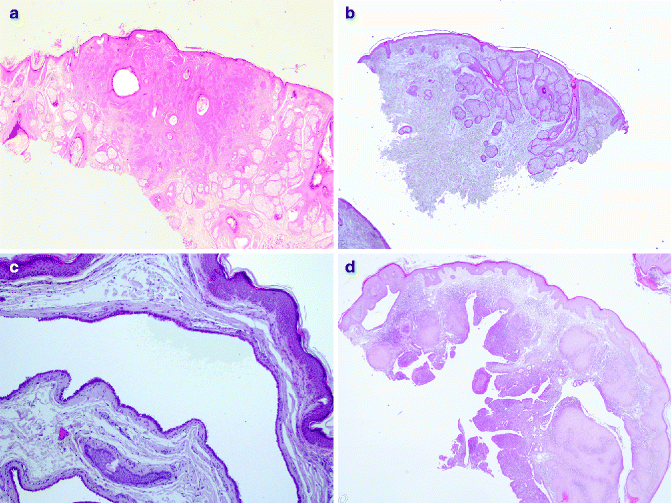

Fig. 6.1
Hamartomatous proliferation: (a) trichofolliculoma (H&E 2×), (b) sebaceous nevus (H&E 2×), (c) hidrocystoma (H&E 10×), (d) SCAT (H&E 2×)
The inherited or acquired basaloid follicular hamartomas histologically consist of anastomosing strands, branching cords of small basaloid cells admixed with squamous cells, deriving from hair follicles. It is often associated to other clinical conditions such as myasthenia gravis, basal-cell carcinoma (BCC) and systemic lupus erythematosus and alopecia [7–9]. The basaloid epidermal proliferation is morphologically similar to BCC, but it overlies dermatofibroma [10]. The group of trichofolliculoma-like lesions includes very similar hamartomatous proliferations. Trichofolliculomas develop at any age in the face and it consists of keratin-filled, unilocular or multilocular cysts open to the epidermis; from this main cavity, variable numbers of sebaceous glands bud out and branches develop [11]. In the sebaceous trichofolliculoma, the tumour is characterised by sebaceous gland opening to the cyst cavity [12]. Hamartomatous cysts are not open to the overlaying epidermis in the folliculosebaceous cystic hamartoma [13]. Pilar sheath acanthoma histologically consists of superficial broad invagination producing a crater-like cavity [6]. Finally, trichodiscoma/fibrofolliculoma is characterised by dilated follicular infundibulum, from which there is a proliferation of thin, trabeculated strands of basophilic epithelium within fibrous stroma [14]. Sebaceous hyperplasia is constituted by immature sebocytes organised in five or more lobules of a sebaceous gland opening into a single dilated follicular infundibulum. It is observed in middle-aged and elderly individuals, generally to the forehead and cheeks, as a small, yellow papule. Multiple sebaceous lesions (hyperplasia, adenoma, sebaceoma and carcinoma) can be associated to Muir–Torre syndrome [15]. The nevus sebaceous of Jadassohn (NSJ) is observed at birth as solitary yellowish, waxy hairless plaques and as nodular lesion in adults. It generally occurs in the head and neck region. Histologically it is characterised by mature elements of all lines of sebaceous differentiation and shows a wide range of morphological features. A variety of benign and malignant adnexal tumours have been described in association with NSJ. In a recent study of 596 cases of NSJ, malignancies were encountered in 1 % of cases, whereas benign skin tumours were identified in 13.6 % of cases of NSJ [16]. Allelic deletions of the human homologue of the Drosophila-patched gene are described in NSJ, justifying malignant tumours development within the lesions [17].
Benign and Malignant Pilosebaceous Tumours
Trichoepithelioma, Trichoadenoma and Desmoplastic Trichoepithelioma
Trichoepithelioma generally occurs in the head and neck region, as sporadic or as autosomal dominant disorder with multiple lesions (Fig. 6.2a). The gene related to Multiple Familial Trichoepithelioma (MFT) is probably CYLD located in the short arm of chromosome 9, in which several tumour-suppressor genes are located [18–20]. Histologically, trichoepithelioma is characterised by nests of uniform basaloid cells with peripheral palisading and formation of keratin cysts [21]. Rarely malignant trichoepithelioma have been reported [22]. Trichoadenoma is a rare benign follicular neoplasm characterised by proliferation of follicular germ with predominant keratin cysts and inconspicuous basaloid cells [6]. Desmoplastic trichoepithelioma (DTE) variant is characterised by compressed strands of basaloid germinative epithelium, with or without small horn cysts [23].
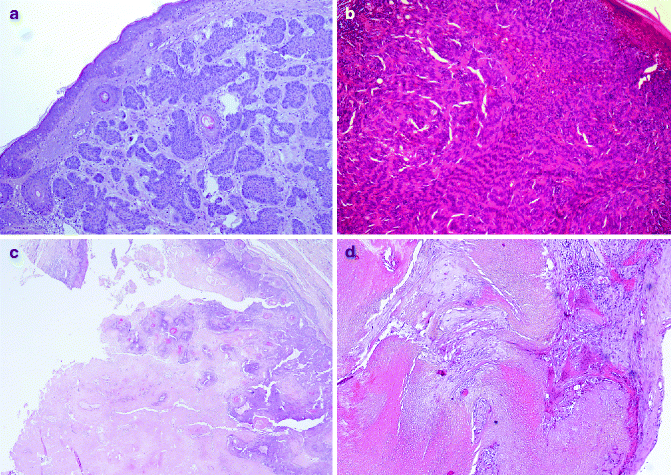

Fig. 6.2
Pilosebaceous benign tumors: (a) trichoepithelioma (H&E 10×), (b) trichoblastoma (H&E 20×), (c) pilar tumour (H&E 10×), (d) pilomatricoma (H&E 20×)
Trichoblastoma
Trichoblastoma develops in elderly individuals, commonly in the head and neck region [24]. Histologically it consists of nodules of cords and nests formed of solid basaloid germinative epithelial cells with peripheral palisading (Fig. 6.2b). Numerous melanocytes and melanin pigment are also frequently seen within the epithelial component [16]. Trichoblastic carcinoma is rarely described; it is a high-cellularity lesion with infiltration of underlying muscles [25].
Trichilemmoma
Trichilemmomas arise from the hair follicle outer root sheath, mainly of the bulb region. It develops in the face of adult individuals as solitary, small skin-coloured papule verrucous lesion. Multiple facial trichilemmomas are observed in Cowden syndrome (multiple hamartoma syndrome) [26] (Fig. 6.3a). Histologically, it consists of well-circumscribed and symmetric lesion connected to the epidermis, which show verruca vulgaris-like changes. Two forms are described: the prototypical early lesion with hyperplasia of the infundibular epithelium, with differentiation towards the outer root sheath, and the fully developed lesions with lobules of uniform cells with clear cytoplasm and peripheral palisading pattern lobules. A desmoplastic trichilemmoma is also described [27]. Finally, very rare cases of trichilemmal carcinoma have been described [28] (Fig. 6.3b, c).
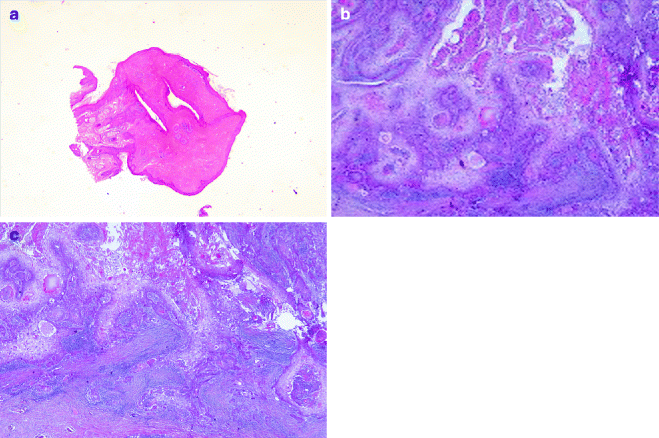

Fig. 6.3
Trichilemmal tumours: (a) trichilemmoma (H&E 2×), (b) trichilemmal carcinoma (H&E 20×), (c) trichilemmal carcinoma metastasis (H&E 20×)
Proliferating Trichilemmal Cyst (Pilar Tumour)
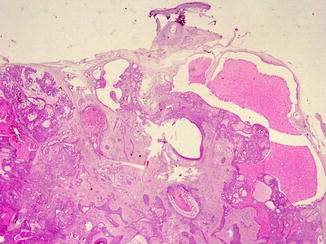
Proliferating trichilemmal cyst (PTC) occurs on the scalps of elderly women, as nodulo-cystic lesion, usually ranging from 1 to 10 cm in diameter [29]. Histologically, PTC develops in the deep dermis and subcutaneous tissue, as well-defined, lobulated, solid and cystic mass of proliferating squamous epithelium, surrounded by thick hyalinised basement membrane (Fig. 6.2c). Malignant transformation is rarely described in PTC [30].
Pilomatricoma (Pilomatrixoma)
Pilomatricoma, or calcifying epithelioma of Malherbe, is a benign dermal and/or subcutaneous tumour, most commonly observed in children and adolescents. Pilomatricoma is generally observed in the head and neck region and upper extremities. Multiple pilomatricomas are associated with different clinical syndromes, such as myotonic dystrophy Rubinstein–Taybi syndrome and Turner syndrome [31, 32]. Activation mutations of b-catenin are found in pilomatricoma [33]. Histologically pilomatricoma shows a well-circumscribed nodular lesion in the dermis and/or subcutis, surrounded by fibrous stroma, with early lesions characterised by basaloid cells lining a cystic cavity and contiguously transformed into nucleated shadow/ghost cells admixed with keratin and older lesions characterised by prominent shadow cell component, keratin debris and dystrophic calcification (Fig. 6.2d). Proliferating variant is characterised by prominent cellularity and scanty shadow cells. Pilomatrix carcinoma is a very rare tumour with high risk of local recurrence [34].
Sebaceous Neoplasms
Sebaceous adenoma occurs as dermal nodule. Histologically it shows lobules of predominate mature sebaceous cells and is peripherally located in germinal basaloid epithelial cells, with the absence of central draining duct. Sebaceoma is a histological variant of SA, with a prevalence of germinal basaloid epithelial cells and ductal differentiation. Also squamous metaplasia may rarely be seen.
Sebaceous carcinoma is frequently observed in the eyelid (ocular, palpebral sebaceous carcinoma), deriving from modified sebaceous gland, such as Meibomian glands or glands of Zeis, sometimes in the context of Muir–Torre syndrome. It is characterised by local recurrence and distant metastases. Histologically, asymmetry; poor circumscription; infiltrative growth pattern and preponderance of atypical, pleomorphic, basaloid cells arranged in solid sheets; and high mitotic activity characterise sebaceous carcinoma. Scattered sebocytes are often present. Tumour necrosis is related to poor prognosis [35]. Neoplastic cells are immunopositive for cytokeratin, low molecular CK, EMA, CA 15.3 and androgen receptor protein and immunonegative for CEA, S100 protein and GCDFP-15.70 [36]. Variants of sebaceous carcinoma are basaloid, spindle, squamoid cells and dedifferentiated (pleomorphic) (Fig. 6.4).
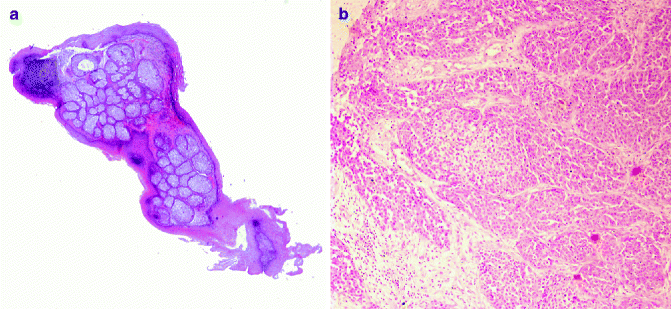

Fig. 6.4
Sebaceous neoplasm: (a) sebaceous adenoma (H&E 2×), (b) sebaceous carcinoma (H&E 20×)
Sweat Gland Tumours
Histology of Sweat Glands (SG)
Eccrine SGs are widely distributed almost everywhere in the skin, with higher concentration in palms, soles and forehead; they develop from the fetal epidermis and are composed of three segments: intraepidermal duct (acrosyringium), intradermal duct and secretory portion. The ducts are lined with two layers of cells, whilst the secretory portion is composed of one layer of secretory cells, surrounded by myoepithelial cells.
Apocrine SGs are mainly localised in the axillary, groin and anogenital regions and, as modified glands, in the external ear canal (ceruminous gland), in the eyelid (Moll’s glands) and in the breast (mammary glands). They develop as epithelial buds from the outer sheath of the hair germ (and therefore the apocrine duct usually leads to a follicular infundibulum), becoming functional only during puberty. The apocrine basal coils are located in the subcutaneous fat and are composed entirely of secretory cells; the ductal and secretory portions show the same composition of eccrine glands.
Apoeccrine SGs are mostly found in the axillary region, and within lesions of NSJ. Their presence explains the existence of some adnexal tumours having both eccrine and apocrine differentiation, including syringocystadenoma papilliferum (SCAP) and Fox–Fordyce disease. Some authors believe that these glands are synonymous to cutaneous mammary-like glands (MLG), which are now recognised as normal component of the skin in the anogenital region, including the perianal skin. MLG are characterised by features of eccrine, apocrine and specialised mammary glands.
General Consideration for Diagnosis of Cutaneous Sweat Gland Tumours
Cutaneous sweat gland tumours (CSGT) are uncommon, with a wide histological spectrum, complex classification and many different terms often used to describe the same tumour.
Historically CSGTs have been divided into apocrine and eccrine, but the histologic distinction is not always easy, and therefore, neoplasms with ductular differentiation often have debatable histogenesis; moreover, the description of apoeccrine glands and the existence of lesions with composite/mixed differentiation make the matter further complicated. Table 6.5 shows the classification of CSGT according to the current opinion about the predominant ‘accepted’ origin.
Table 6.5
Sweat gland tumours origin classification
Origin/Differentiation | Hamartomas/benign neoplasms | Malignant neoplasms |
|---|---|---|
Eccrine and apocrine | Hidrocystoma | |
Apocrine/eccrine nevus | ||
Chondroid syringoma (mixed tumour) | ||
Eccrine | Poroma | Porocarcinoma |
Hidradenoma | Hidradenocarcinoma | |
Spiradenoma | Spiradenocarcinoma | |
Cylindroma | Malignant cylindroma | |
Syringoma | Syringoid carcinoma | |
Microcystic adnexal carcinoma* | ||
Aggressive Digital Papillary Adenocarcinoma* | ||
Adenoid cystic carcinoma | ||
Mucinous Carcinoma* | ||
Apocrine | Syringocystadenoma papilliferum | Syringocystadenocarcinoma |
Hidradenoma papilliferum | Apocrine carcinoma | |
Extramammary Paget’s disease |
CSGT Classification
Benign/Hamartomatous Lesions
Hidrocystoma are cystic proliferation of SG, relatively rare, with predilection for the face and neck of middle-aged or older individuals. They have either apocrine or eccrine differentiation. The hidrocystomas represent retention cysts deriving from the gland duct [37]. Histologically hidrocystomas can be a uni- or multilocular lesion, usually lined by a double layer of epithelial cells. The inner layer consists of columnar cells with apical snouts or of flattened epithelium (Fig. 6.1c).
Syringocystadenoma papilliferum (SCAP) usually affects the young adults and occurs as a solitary or, less commonly, multiple lesions, most commonly in the face and scalp; they can occur in a pre-existing NSJ and rarely can be associated with other eccrine or apocrine tumours. Mutations in tumour-suppressor gene p16 may contribute to the pathogenesis of SCAP [38]. Histologically, SCAP is characterised by multiple epidermal invaginations and papillae with a fibrovascular core and lined by two layers of epithelial cells (Fig. 6.1d). The luminal columnar cells exhibit decapitation secretion, as typical apocrine features.
Benign and Malignant Tumours
The differences among the benign CSGTs have only an academic value, since the precise categorisation of neoplasms rarely impacts on the clinical management. Indeed the surgical excision is generally curative.
SGs carcinomas are relatively less common than benign tumours, deriving mainly from eccrine SGs. They occur in adults, but they have also been reported in children. Malignant CSGTs arise mainly as de novo neoplasm, although malignant transformation of a pre-existent benign CSGTs has been described, usually as high-grade carcinoma [39]. Histologically, most malignant CSGTs preserve some features of their benign counterparts; clues for histologic distinction between benign and malignant tumours are irregularly permeating growth, nuclear atypia, perineural/vascular invasion by tumour cells, and foci of spontaneous tumour necrosis. Mitotic activity is not diagnostically helpful, being observed in benign tumours, too [40]. In addition to the malignant counterparts of the various types of CSGTs, several other distinctive CSG carcinomas occur, i.e. microcystic adnexal carcinomas, aggressive digital papillary adenocarcinoma, adenoid cystic carcinoma and primary cutaneous mucinous carcinoma of the skin.
Herein we proposed the most common CSG tumours, accounting for each group both benign tumour and malignant counterpart.
Eccrine Tumours
Poroma/Porocarcinoma
Poromas (acrospiromas) are neoplasms with excretory eccrine duct differentiation; they usually arise in acral sites, particularly palms and soles. Histologically poroma consists of a proliferation of basaloid uniform tumour cells organised in ductal structures and occasional cysts, extending from the lower epidermis into the dermis (Fig. 6.5a).
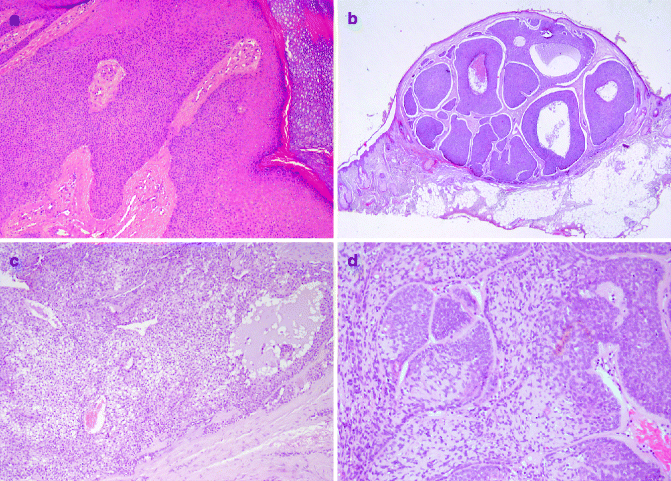

Fig. 6.5
Benign sweat gland tumours: (a) poroma (H&E 20×), (b) hidroadenoma (H&E 2×), (c) spiradenoma (H&E 20×), (d) cylindroma (H&E 20×)
Porocarcinoma is usually found in the lower extremities. It is characterised by asymmetrical, solid, growth pattern nodules of basaloid tumour cells, resembling those of poroma, but shows varying degrees of cytonuclear atypia, hyperchromatic nuclei and prominent nucleoli. Porocarcinomas are aggressive tumours with potential local recurrence and metastasis. Indeed more than 14 mitoses per high power field, tumour depth of 7 mm and presence of lymphovascular invasion are associated with a more aggressive clinical course [24, 41]. Mutation of p53 gene with loss of its suppressor function has been widely reported [42].
Hidroadenoma/Hidradenocarcinoma
Hidradenoma is generally considered as an eccrine neoplasm. Clinically its presentation is a solitary, skin-coloured lesion and occurs more commonly in females. It has variable histomorphological patterns and consists of nests and nodules of small, monomorphous epithelial cells, with small ductular lumens, developing in the upper dermis. The lesion is characterised by pushy, but well-circumscribed, peripheral border (Fig. 6.5b). Recurrence of not fully excised hidradenoma is approximately observed in 12 % of all cases [40]. Most cases of hidradenocarcinoma arise de novo. Histologically tumour cells have similar features of hidradenoma, with cytonuclear atypia and foci of necrosis. Mitotic figures may be focally prominent. This carcinoma may metastasise [43].
Spiradenoma and Spirocarcinoma
Spiradenomas, derived from straight intradermal eccrine duct, usually present as a solitary, painful, nodular lesion in young adults, with a predilection of the trunk and upper extremities. They can be rarely observed in Brooke–Spiegler syndrome, a rare autosomal dominant inherited disorder, characterised by the development of multiple skin adnexal tumours [44]. Microscopically, spiradenomas are lobulated mass composed of multiple nests of small basophilic cells, admixed with squamoid cells and clear cells, with hyalinised basement membrane, and ductal differentiation (Fig. 6.5c).
Spiradenocarcinoma is a very rare malignant neoplasm, resulting from malignant transformation of spiradenoma, with loss of nodular growth pattern, infiltrative borders, cytonuclear pleomorphism and tumour necrosis. TP53 mutations have been identified in carcinomatous portion of these tumours [45].
Cylindroma and Malignant Counterpart
Cylindromas usually affect adult females, mainly involving the scalp and the face. They can be a solitary lesion or less commonly multiple nodules coalescing tumour (turban tumour), mainly observed in familial cylindromatosis and Brook–Spiegler syndrome. The pathogenesis of cylindroma is associated with mutation in the cylindromatosis tumour-suppressor gene (CYLD1), on chromosome 16q12–1319. Histologically, these tumours are characterised by numerous well-defined nodules of epithelial cells, lined by thick PAS positive basement membrane-like, hyalinised material, with a characteristic ‘jigsaw puzzle’ pattern (Fig. 6.5d).
Malignant cylindroma is a rare tumour arising from pre-existing benign cylindroma, characterised by infiltrative growth pattern with cytonuclear pleomorphism and loss of jigsaw appearance, hyaline sheath and peripheral palisading. The tumour has an aggressive behaviour [44].
Syringoma and Malignant Counterpart
Syringomas are small lesions, arising from the straight segment of the intradermal eccrine sweat duct, always multiple of the lower eyelid and upper cheeks. They are found more often in women and in adolescence or early adulthood and are constituted of tubules.
Chondroid syringoma is an uncommon tumour generally occurring in the head and neck region, most commonly the nose, cheek and upper lip. Histologically, it is a mixed tumour having both epithelial and mesenchymal stromal components. Thus, it is constituted of cords, ducts or tubules of bland epithelial cells, immersed in chondromyxoid stroma. Chondroid syringoma is classified into apocrine and eccrine types; in the apocrine type, lesions incorporating areas with pilar and sebaceous differentiation have been described suggesting that some tumours are best viewed as hamartomatous lesions of the pilosebaceous/apocrine unit [46] (Fig. 6.6). Rare malignant mixed tumours have been described, generally developing de novo [47, 48]. Histological diagnosis is foremost based on the biphasic nature of the neoplasm and an origin from MMT myoepithelial cells appears to be most plausible.