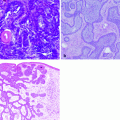
Fig. 32.1
Preoperative lymphoscintigraphy allows to identify nodal basins involved in the lymphatic spread of cancer. The arrows identify sentinel node in anterior and latenal views
Vital Blue Dye
Vital blue dyes complement lymphoscintigraphy by helping to visualize the SNs during dissection. Isosulfan blue (Lymphazurin) and patent blue V are both effective. Methylene blue has also been used because of its greater availability; however, it is less effective at highlighting lymphatic channels and has been associated with soft tissue necrosis. Vital blue dye is injected intradermally using a tuberculin syringe (27 G) around the primary lesion or biopsy scar. The volume of dye depends on the anatomic site: 1–2 ml for sites on the trunk, extremity, and scalp, but only 0.5–0.75 ml on the face because of wider diffusion, particularly around the eye. Injection into the subcutaneous tissue should be avoided because lymphatic density is lower and the dye may not migrate. Also, subcutaneous tissues have separate and distinct lymphatic channels and therefore may lead to incorrect SN identification. The time interval from injection of blue dye to surgical dissection depends on the distance from the primary lesion to the regional nodal basin that will be explored. Lymphatic flow is fastest for the distal limbs and slowest for the head-neck region. Typically within 5–15 min, the blue dye has entered the SN and washout from the node is evident after approximately 45 min [28].
A contraindication to blue dye injection is known as allergic reaction to blue dye; anaphylaxis is rare but has been reported. As mentioned above, pregnancy is a relative contraindication because the long-term toxicity to the fetus is unknown. Adverse side effects to blue dye include allergic reactions (“blue hives”), which are seen in up to 2 % of cases and may be influenced by blue dye volume. Other side effects are blue-colored urine for up to 24 h following administration and a factitious drop in intraoperative oxygen saturation measured by pulse oximetry.
Handheld Gamma Probe
The handheld gamma probe is a radiation detector that provides a count rate from gamma rays. It is an effective tool that facilitates the intraoperative identification of radioactive lymph nodes that may or may not be blue stained (Fig. 32.2). If SN biopsy is performed shortly after preoperative lymphoscintigraphy, the handheld gamma probe can be used to identify residual radiotracer in the SN. After a few hours, radioactivity decreases in the SN but can be measured in second-tier nodes. Because the radioisotope has a half-life of approximately 5–6 h, SN biopsy can also be performed the day after lymphoscintigraphy. In this case, radioactive counts will be lower and the SN sometimes may be more difficult to identify.


Fig. 32.2
The handheld gamma probe routinely used in our operative rooms
Before the skin is incised, the gamma probe should be moved systematically over the lymphatic basin to confirm the accuracy of the marked skin site (Fig. 32.3); if the patient’s position during surgery differs from that during lymphoscintigraphy, then the skin marks may no longer approximate the location of the SN. Because the preoperative lymphoscintigraphic image given to the surgeon represents only a snapshot of a dynamic process, potential drainage basins (including ectopic locations such as the intermuscular triangle of the back) should be checked with the probe to confirm the absence of radioactivity.
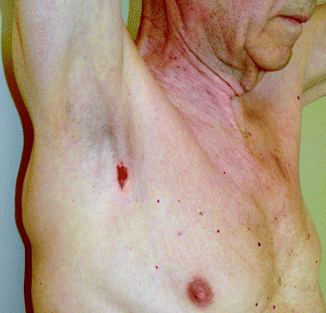

Fig. 32.3
The marked skin site (right axillary sentinel node biopsy) needs to be confirmed in the chosen operative position before skin incision
The general function of the probe can be crudely checked by pointing the probe at the injection site to test for a response. The nuclear medicine report and the skin mark act as reference point for the SN search. First, set initial count range to the lowest setting and volume to a clearly audible setting. Correct range is important because an insufficient pitch variation can prevent identification of some radioactive nodes. Record the count activity at the injection site and the background count activity around the basin. Start at the position in the basin closest to the injection site, place the probe perpendicular to the skin surface, and scan in a back and forth pattern moving away from the injection site. When count activity increases, localize the hot spot by scanning perpendicular to the initial scan line. The speed of scanning should be approximately 1–5 cm/s [29].
The distance between the injection site and the drainage basin can affect hot spot identification: the closer the injection site is to the drainage basin, the more likely that background counts will be constant because radioactivity “shines through” from the primary injection site. If the injection site is within the probe’s field, counts will be falsely elevated. As a practical rule, we suggest to always perform radicalization of the primary site, with excision margins indicated by official guidelines, prior to identifying the SN.
Surgical Technique
After lymphoscintigraphy, the patient is transported to the operative room; anesthesia is induced at the discretion of the anesthesiologist in consultation with the surgeon. Many surgeons still prefer adjunctive intradermal injection of blue dye (approximately 1 ml) at the site of the lesion. A handheld gamma probe is used to identify “hot spots,” which will indicate areas where SLNs are and to place the skin incision.
The key principle of the procedure is to remove all SNs avoiding unnecessary tissue trauma, with minimal dissection (which is facilitated either by the blue dye trace or by the count rate of the gamma probe). The probe is in fact usually placed on the tissue and pointed in different directions to ensure the radioactivity pathway. Meticulous, gentle dissection should avoid transecting the lymphatics, because disruption of these vessels leads to contamination of radiotracer and dye and to an increased risk of seroma. Electrocautery is used to dissect through the subcutaneous tissue and fascia. Blunt dissection towards the hot node is preferred using a tonsil clamp. Afferent and efferent lymphatic and blood vessels are ligated with clips and/or suture. Care is taken to neither crush nor cauterize the specimen.
After a node is dissected free, the radioactivity of this node is checked by placing it on a surface away from the patient to avoid interference. The operative site is finally tested for remaining activity. The decision to remove eventual further nodes will depend on the persistence of radioactivity in the operative field. Lymphatic vessels and lymph nodes may be replaced by tumor and fail to take up mapping agents; therefore, the operative site should be inspected and palpated for clinically suspicious lymph nodes. All such lymph nodes should be removed.
Lymphatic mapping in the groin and axilla is associated with high rates of SN identification and low rates of false-negative SNs (1 % for the groin and 5 % for the axilla). There is no consensus on whether to excise external iliac or obturator SNs identified by lymphoscintigraphy; we recommend removing them through a retroperitoneal approach if the lymphoscintigram demonstrates direct drainage to these nodes from the primary site on the skin.
Lymphatic mapping in the head and neck region requires much more experience for comparable success, because this area has a dense and complex pattern of communicating lymphatic channels, often with multiple SNs. Though the lymphoscintigram may indicate only one hot node, there may actually be multiple hot nodes in a cluster that appear as one due to the limited resolution of the gamma camera. Reported false-negative rates for the head-neck region have been as high as 15 %. For these reasons, we agree with many authors that the head-neck region is the most difficult site to map.
Failure rate to identify a sentinel lymph node is <5 %. If no SN can be found, a complete lymph node dissection should be considered for high-risk primary lesions.
Frozen Section
Sentinel nodes are fixed in 10 % neutral, buffered formalin, and, after fixation, they are bisected through the hilum (if identifiable) or through the long axis of the node. If the halves are thicker than 2 mm, the slices are further trimmed to provide additional blocks of 2 mm. If sentinel nodes are found to be free from tumor after the initial histologic examination, step-serial sections are prepared at an additional six levels in the block at intervals of approximately 150 μm.
For permanent sectioning, it’s possible to perform hematoxylin and eosin (H&E) staining followed by staining with immunohistochemical (IHC) markers S-100, MART-1, and HMB-45. In addition to IHC evaluation, National Comprehensive Cancer Network (NCCN) guidelines also recommend that multiple sectioning of each node be performed to pick up potential micrometastatic deposits. Consideration can also be given in performing polymerase chain reaction (PCR) evaluation of the SLN.
Postoperative Care
In general, patients undergoing sentinel lymph node biopsy are discharged the same day of the procedure or on postoperative day 1, depending on the treatment of the primary neoplasm and the reconstructive strategy chosen. Drainage in axillary or inguinal cavity after lymph node sentinel biopsy is not mandatory. Sutures are usually removed within 2 weeks postoperative. Extremity elevation and elastic compressive bandage wraps help to prevent lymphedema. These patients are usually advised to wear compression stockings 4–6 weeks postoperatively.
Expected Side Effects and Complications
Although sentinel lymph node dissection with selective lymphadenectomy is less morbid than elective lymph node dissection, as with any invasive procedure, complications do occur. In the Sunbelt Melanoma Trial [30], the overall complication rate was less than 5 % for sentinel lymph node biopsy. The mortality rate was 0 %.
Complications occur more frequently in patients with cardiac disease, obesity, diabetes mellitus, and cigarette smoking. The complication rate has also been reported to increase with the number of sentinel nodes removed.
Immediate and Short-Term Expected Side Effects
Immediate complications of the procedure include anaphylaxis or other allergic reactions to the intradermal injection of blue dye (<1 %) and bleeding. Short-term postoperative complications of the procedure include hematoma, wound infection, seroma, and flap necrosis. Seroma and wound infection are the most common but the incidence rate is under 2 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree