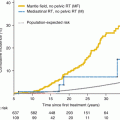
Fig. 19.1
Cumulative incidence of second neoplasms after initial cancer diagnosis (Friedman et al. [3])
Unique associations with specific therapeutic exposures have resulted in the classification of SMNs into two distinct groups: chemotherapy-related myelodysplasia and acute myeloid leukemia (t-MDS/AML) and radiation-related solid SMNs. Characteristics of AML include a short latency (<5 years from primary cancer diagnosis) when associated with topoisomerase II inhibitors vs. AML/MDS that usually occur with a longer latency extending 10–15 years after therapeutic exposure when associated with alkylating agents. Solid SMNs have a well-defined association with radiation and are characterized by a latency that typically exceeds 10 years [3, 5–7]. The most frequently observed solid SMNs include the breast, skin, thyroid, and central nervous system (CNS) tumors and bone/soft tissue sarcomas and carcinomas [8–10] (Table 19.1).
Table 19.1
Characteristics of second cancers among childhood cancer survivors – by primary cancer type
Primary cancer | Secondary cancer | Median latency | Risk factors |
|---|---|---|---|
Hodgkin lymphoma | Breast cancer | 15–20 | Radiation |
Female sex | |||
Myelodysplastic syndrome/acute myeloid leukemia | 3–5 years | Alkylating agents | |
Topoisomerase II inhibitors | |||
Thyroid cancer | 13–15 years | Radiation | |
Younger age | |||
Female sex | |||
Soft tissue sarcoma | 10–11 years | Radiation | |
Younger age | |||
Anthracyclines | |||
Bone tumor | Breast cancer | 15–20 years | Radiation |
Female sex | |||
Myelodysplastic syndrome/acute myeloid leukemia | 3–5 years | Alkylating agents | |
Topoisomerase II inhibitors | |||
Thyroid cancer | 13–15 years | Radiation | |
Younger age | |||
Female sex | |||
Other bone tumors | 9–10 years | Radiation | |
Alkylating agents | |||
Soft tissue sarcomas | 10–11 years | Radiation | |
Younger age | |||
Anthracyclines | |||
Soft tissue sarcoma | Breast cancer | 15–20 years | Radiation |
Female sex | |||
Thyroid cancer | 13–15 years | Radiation | |
Younger age | |||
Female sex | |||
Bone tumor | 9–10 years | Radiation | |
Alkylating agents | |||
Other soft tissue sarcoma | 10–11 years | Radiation | |
Younger age | |||
Anthracyclines | |||
Acute lymphoblastic leukemia | Breast cancer | 15–20 years | Radiation |
Female sex | |||
Brain tumors | 9–10 years | Radiation | |
Younger age | |||
Myelodysplastic syndrome/acute myeloid leukemia | 3–5 years | Alkylating agents | |
Topoisomerase II inhibitors | |||
Thyroid cancer | 13–15 years | Radiation | |
Younger age | |||
Female sex | |||
Bone tumors | 9–10 years | Radiation | |
Alkylating agents | |||
Brain tumor | Other brain tumors | 9–10 years | Radiation |
Younger age | |||
Breast cancer | 15–20 years | Radiation | |
Female sex | |||
Thyroid cancer | 13–15 years | Radiation | |
Younger age | |||
Female sex | |||
Wilms tumor | Breast cancer | 15–20 years | Radiation |
Female sex | |||
Non-Hodgkin lymphoma | Breast cancer | 15–20 years | Radiation |
Female sex | |||
Thyroid cancer | 13–15 years | Radiation | |
Younger age | |||
Female sex |
t-MDS/AML have been reported after successful treatment of Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), acute lymphoblastic leukemia (ALL), and bone/soft tissue sarcomas [5, 8, 11–15] (Table 19.1). The risk of t-MDS/AML is generally low, approaching 2 % at 15 years after conventional therapy [5]. Using the WHO classification, two types of t-MDS/AML are recognized, related closely to the therapeutic exposure (Table 19.2): alkylating agents/radiation and topoisomerase II inhibitors [16]. The alkylating agent-related t-MDS/AML typically develops ~5 years after exposure. Cytopenias are common. Two-thirds of the patients present with myelodysplasia; the remaining present with AML but carry myelodysplastic features. Abnormalities involving chromosomes 5 (−5/del[5q]) and 7 (−7/del[7q]) are frequently seen. AML secondary to topoisomerase II inhibitors presents as overt leukemia, without a preceding myelodysplastic phase. The latency is brief, ranging from 6 months to 5 years, and is associated with balanced translocations involving chromosome bands 11q23 or 21q22. Epipodophyllotoxin-associated t-AML depends more on the schedule of drug administration than total cumulative dose [17].
Table 19.2
Clinical characteristics of treatment-related myelodysplastic syndrome and acute myeloid leukemia
Characteristics | Alkylating agents | Epipodophyllotoxins |
|---|---|---|
Median latency | 4–6 years (range, 1–20) | 1–3 years (range, 0.5–4.5) |
Presentation | Myelodysplasia | Abrupt-onset leukemia – no pre-leukemic phase |
Cytogenetic abnormalities | Loss of genetic material, often from chromosomes 5 and/or 7 | Balanced translocations (often include 11q23) |
Age at onset | Typically older patients | Typically younger patients |
Solid SMNs are clearly related to radiation therapy used to treat the primary cancer and thus will usually arise within the radiation field [5, 6, 8, 18, 19]. However, solid SMNs can also occur outside of radiation fields and also in children treated with chemotherapy alone. The latency for radiation-related solid SMNs usually exceeds 10 years [5, 6, 8, 19]. The risk is highest when radiation exposure occurs at a younger age [5, 7, 19–26] and increases with increasing doses of radiation and with increasing time since radiation [6, 8]. Eighty percent of the entire burden of SMNs is accounted for by radiation-related solid SMNs. Some of the well-established radiation-related solid SMNs include breast cancer, thyroid cancer, CNS tumors, sarcomas, and basal cell carcinomas (BCCs) [3, 5–8, 24, 27].
Breast Cancer
Breast cancer is the most commonly reported second malignancy among female survivors of childhood HL treated with mantle field irradiation (SIR = 24.7, 95 % CI, 19.3–31.0), and the risk remains markedly elevated for many decades after exposure [5, 19, 27–30]. The survivors have up to a 55-fold increased risk of breast cancer compared with the general population, and the cumulative incidence of developing a secondary breast cancer approaches 20 % at 45 years of age [8]. Moreover, 40 % of the patients with radiation-related breast cancer develop contralateral disease, usually within 1–3 years. The incidence is also increased among those exposed to TBI as the only source of radiation to the chest when compared with those who did not receive TBI (17 % vs. 3 %) [31]. The risk of breast cancer increases in a linear fashion with radiation dose, reaching 11-fold for local breast doses of ~40 Gy relative to no radiation [32]. Conversely, a reduction in irradiated breast volume, at least in patients irradiated for Hodgkin lymphoma, decreases the risk for breast cancer. The risk of radiation-related breast cancer declines with age at radiation, such that the relative risks in cancer patients who had received radiation after age 40 years are comparable to those of the general population [33, 34]. There appears to be a protective effect of early menopause induced either by alkylating agents or radiation dose >5 Gy to the ovaries, suggesting that ovarian hormones play an important role in promoting tumorigenesis once an initiating event has been produced by radiation [27, 32, 35, 36].
Thyroid Cancer
Secondary thyroid malignancies, typically papillary carcinoma, are generally associated with radiation exposure to the thyroid gland as part of CNS irradiation, for treatment of CNS leukemia, as part of therapeutic irradiation of cervical lymph nodes in HL patients, or as part of conditioning with TBI for hematopoietic cell transplantation [5, 7, 8, 12, 19]. Thyroid malignancy typically develops 10 or more years from treatment. Veiga et al. analyzed multiple cohorts including CCSS, noting that a linear exponential model best described the relative risk of thyroid cancer; RR increased through 10 Gy (to 13.7 95 % CI: 8.0–24.0) and then reached a plateau until 30 Gy when a downturn in the dose-response relationship was observed[37]. This pattern is influenced by effect modification attributable to chemotherapy (alkylating agents, bleomycin, anthracyclines), which increases the RR for patients receiving <20 Gy. Sex (higher radiation risk among females), age at exposure (higher radiation risk at a younger age at exposure), and time since exposure (higher radiation risk with longer time) are significant modifiers of the radiation-related risk of thyroid cancer [38]. HCT recipients are at a 3.3-fold increased risk of thyroid cancer, when compared with age- and sex-matched general population [39]. Younger age at HCT (<10 years), neck radiation, female sex, and chronic GvHD are associated with an increased risk of thyroid cancer. Thyroid cancer usually develops after a latency of ~10 years, and, as is true of de novo thyroid malignancy, the long-term outcome for survivors diagnosed with a secondary thyroid malignancy is excellent.
Central Nervous System Tumors
Brain tumors develop after cranial radiation for histologically distinct brain tumors [6] or for prophylaxis or treatment of CNS leukemia [7, 12, 19]. The risk is 16.9-fold higher than that of the general population for ALL survivors and is 14.2-fold increased for brain tumor survivors. Histologically, radiation-related late-occurring CNS tumors include high-grade gliomas (glioblastomas and malignant astrocytomas), peripheral neuroectodermal tumors, ependymomas, and meningiomas [7, 13, 40, 41]. Gliomas are diagnosed a median of 9 years from radiation; for meningiomas, the latency is longer (median latency = 17 years) [6]. Radiation exposure is associated with increased risk of both subsequent glioma (OR = 6.8) and meningiomas (OR = 9.9). The dose-response for the excess relative risk is linear. For gliomas, the excess relative risk per Gy is highest among children exposed at less than 5 years of age.
Sarcomas
The risk of sarcoma after an initial diagnosis of childhood cancer is reported to be ninefold that in the general population; the risk is particularly elevated after a primary soft tissue sarcoma (24.7-fold), bone tumor (10.6-fold), HL (11.7-fold), or renal tumors (14.6-fold) [42]. Patients with heritable retinoblastoma are at a particularly increased risk of radiation-related sarcomas (13.7-fold increased risk) [43]. Sarcomas develop within the radiation field after a latency of ~10 years.
Carcinomas
With extended follow-up of cohorts of young survivors, increased risks of common adult carcinomas, including colorectal, lung, and stomach, have emerged, and these cancers are being diagnosed at younger ages than observed in the general population [8, 27, 44–46]. In a large population-based study, breast, lung, and gastrointestinal cancers accounted for almost two-thirds of the estimated excess number of cases [47]. In another population-based cohort of 5-year survivors, the greatest excess risk associated with SMNs among survivors older than 40 years of age was for digestive and genitourinary neoplasms [2]. The risk of carcinomas (other than breast, thyroid, skin) is sixfold higher than that expected [48]. The most common sites are the head and neck (mostly parotid gland), gastrointestinal tract, female genitourinary tract, and kidney. The risk is highest among survivors of neuroblastoma and soft tissue sarcoma; the risk is also elevated for patients who have received radiation therapy.
Skin Cancer
19.2 Pathogenesis of Subsequent Malignant Neoplasms
Although the role of chemotherapy and radiation in the development of second primary cancers is well established, the observed interindividual variability in risk suggests a role for genetic variation in the susceptibility to genotoxic exposures. The risk of chemotherapy- or radiation-related SMNs could potentially be modified by mutations in high-penetrance genes that lead to serious genetic diseases, e.g., Li-Fraumeni syndrome [50] and Fanconi anemia [51–54]. However, the attributable risk is expected to be very small because of the extremely low prevalence of the high-prevalence genes. The interindividual variability in the risk of SMNs is more likely related to common polymorphisms in low-penetrance genes that regulate the availability of active drug metabolite or those responsible for DNA repair. Genetic variation contributes 20–95 % of the variability in cytotoxic drug disposition [55]. Polymorphisms in genes involved in drug metabolism and transport are relevant in determining disease-free survival and drug toxicity [56]. Variation in DNA repair plays a role in susceptibility to de novo cancer [57–61] and likely modifies SMN risk after exposure to DNA-damaging agents, such as radiation and chemotherapy. Genetic predisposition and its interaction with therapeutic exposures can potentially exacerbate the toxic effect of treatment on normal tissues.
In order to understand the pathogenesis of SMNs, it is imperative to understand individual variability in the internal dose of the therapeutic agent, the alterations induced by the therapeutic agent to the structure or function of the tissue, and the consequent development of preclinical disease. Understanding the underlying etiopathogenetic pathways that lead to SMNs is critical in developing targeted prevention and intervention strategies, optimizing risk-based health care of cancer survivors, and improving quality of life.
Drug Metabolism
Metabolism of genotoxic agents occurs in two phases. Phase I involves activation of substrates into highly reactive electrophilic intermediates that can damage DNA – a reaction principally performed by the cytochrome p450 (CYP) family of enzymes. Phase II enzymes (conjugation) function to inactivate genotoxic substrates. The phase II proteins comprise the glutathione S-transferase (GST), NAD(P)H:quinone oxidoreductase-1 (NQO1), and others. The balance between the two sets of enzymes is critical to the cellular response to xenobiotics; e.g., high activity of phase I enzyme and low activity of a phase II enzyme can result in DNA damage from the excess of harmful substrates. The xenobiotic substrates of CYP proteins include cyclophosphamide, ifosfamide, thiotepa, doxorubicin, and dacarbazine [62]. The CYPs transfer singlet oxygen onto their substrates creating highly reactive intermediates which, unless detoxified by phase II enzymes, have a strong ability to damage DNA [63]. The expression of these enzymes is highly variable among individuals because of several functionally relevant genetic polymorphisms. GSTs detoxify reactive electrophiles via conjugation to reduced glutathione, preventing damage to DNA. Polymorphisms exist in cytosolic subfamilies: μ [M], π [P], θ [T], and others. GSTs detoxify doxorubicin, lomustine, busulfan, chlorambucil, cisplatin, cyclophosphamide, melphalan, etc. [64]. Quinone oxidoreductase NQO1 uses the cofactors NADH and NADPH to catalyze the electron reduction of its substrates, produces less reactive hydroquinones, and therefore prevents generation of reactive oxygen species and free radicals which may subsequently lead to oxidative damage of cellular components. Using a candidate gene approach, investigators have examined the association between polymorphisms in the glutathione S-transferase genes (GSTM1, GSTT1, and GSTP1) and t-MDS/AML [65]. Individuals with at least one GSTP1 codon 105 Val allele were significantly overrepresented in t-AML cases compared with de novo AML cases (OR = 1.81, 95 % CI, 1.11–2.94). Also, relative to de novo AML, the GSTP1 codon 105 allele occurred more often among t-AML patients with prior exposure to chemotherapy (OR = 2.66, 95 % CI, 1.39–5.09), particularly among those with prior exposure to known GSTP1 substrates (OR = 4.34, 95 % CI, 1.43–13.20) and not among t-AML patients with exposure to radiation alone. While the genes implicated carried biological plausibility, they were not replicated in this study. Furthermore, the comparison groups consisting of healthy controls and patients with de novo AML could compromise the observations.
DNA Repair
DNA repair mechanisms protect somatic cells from mutations in tumor suppressor genes and oncogenes that can lead to cancer initiation and progression. An individual’s DNA repair capacity appears to be genetically determined [66]. A number of DNA repair genes contain polymorphic variants, resulting in large interindividual variations in DNA repair capacity [66]. Even subtle differences in an individual’s DNA repair capacity may be important in the presence of large external influences such as chemotherapy or radiotherapy. Individuals with altered DNA repair mechanisms are likely susceptible to the development of genetic instability that drives the process of carcinogenesis as it relates to both chemotherapy-related t-MDS/AML and radiation-related solid SMNs.
Mismatch repair (MMR) functions to correct mismatched DNA base pairs that arise as a result of misincorporation errors that have avoided polymerase proofreading during DNA replication [67]. Defects in the MMR pathway result in genetic instability or a mutator phenotype, manifested by an elevated rate of spontaneous mutations characterized as multiple replication errors in simple repetitive DNA sequences (microsatellites) – functionally identified as microsatellite instability (MSI). Approximately 50 % of t-MDS/AML patients have MSI, associated with methylation of the MMR family member MLH1 [68, 69], low expression of MSH2 [70], or polymorphisms in MSH2 [71–74]. The appearance of MMR-deficient, drug-resistant clones during genotoxic treatment for a primary cancer could be a vital factor in SMN susceptibility, particularly because the mutator phenotype (inherent of MMR-deficient cells) would be expected to accelerate the accumulation of further mutations and eventually SMN initiation. In addition, loss of MMR may result in deregulation of homologous recombination repair and consequent chromosomal instability [75].
Double–strand breaks (DSBs) in DNA may lead to loss of genetic material, resulting in chromosomal aberrations. High levels of DSBs arise following ionizing radiation and chemotherapy exposures. Cellular pathways available to repair DSBs include homologous recombination (HR), non-homologous end-joining (NHEJ), and single-strand annealing [76]. HR uses the second, intact copy of the chromosome as a template to copy the information lost at the DSB site on the damaged chromosome – a high-fidelity process. RAD51 is one of the central proteins in the HR pathway, functioning to bind to DNA and promote ATP-dependent homologous pairing and strand transfer reactions [77, 78]. RAD51-G-135C polymorphism is significantly overrepresented in patients with t-MDS/AML compared with controls (C allele: OR = 2.7) [79]. XRCC3 also functions in the HR DSB repair pathway by directly interacting with and stabilizing RAD51 [80, 81]. XRCC3 is a paralog of RAD51, also essential for genetic stability [82, 83]. A polymorphism at codon 241 in the XRCC3 gene results in a Thr→Met amino acid substitution [84]. The variant XRCC3–241Met allele has been associated with a higher level of DNA adducts compared with cells with the wild-type allele, implying aberrant repair [85], and has also been associated with increased levels of chromosome deletions in lymphocytes after exposure to radiation [86]. Although XRCC3–Thr241Met was not associated with t-MDS/AML (OR = 1.4, 95 % CI, 0.7–2.9), a synergistic effect resulting in an eightfold increased risk of t-MDS/AML (OR = 8.1, 95 % CI, 2.2–29.7) was observed in the presence of XRCC3–241Met and RAD51–135C allele in patients with t-MDS/AML compared with controls [79]. NHEJ pathway joins broken DNA ends containing very little homology. This process is not always precise and can result in small regions of non-template nucleotides around the site of the DNA break, potentially relevant in MLL-translocation associated with t-MDS/AML. Many of the translocation junctions have been sequenced and found to contain regions of microhomology consistent with the operation of the NHEJ pathway, and an impairment of this pathway may modulate t-MDS/AML risk [87].
Base excision repair (BER) pathway corrects individually damaged bases occurring as a result of ionizing radiation and exogenous xenobiotic exposure. The XRCC1 protein plays a central role in the BER pathway and also in the repair of single-strand breaks, by acting as a scaffold and recruiting other DNA repair proteins [88, 89]. The protein also has a BRCA1 C-terminus (BRCT) domain – a characteristic of proteins involved in DNA damage recognition and response. The presence of variant XRCC1-399Gln has been shown to be protective for t-MDS/AML [90] and BCC [91].
Nucleotide excision repair (NER) removes structurally unrelated bulky damage induced by radiation and chemotherapy. The NER pathway is linked to transcription, and components of the pathway comprise the basal transcription factor IIH complex (TFIIH), which is required for transcription initiation by RNA polymerase II. One of the genes involved in the NER pathway (ERCC2) is a member of the TFIIH complex. The polymorphic Gln variant (ERCC2 Lys751Gln) is associated with t-MDS/AML [92].
Results from studies examining genetic susceptibility in the development of SMNs are summarized in Table 19.3; some of these studies are highlighted below.
Table 19.3
Role of genetic susceptibility in the development of treatment-related adverse events
Study | GWAS vs. candidate gene | Study design
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|