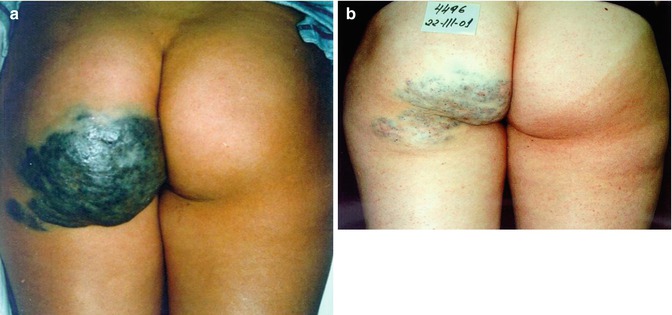
Fig. 34.1
Limited venous malformation of the right forehead. (a) Before and (b) 6 months after sclerotherapy with microfoam
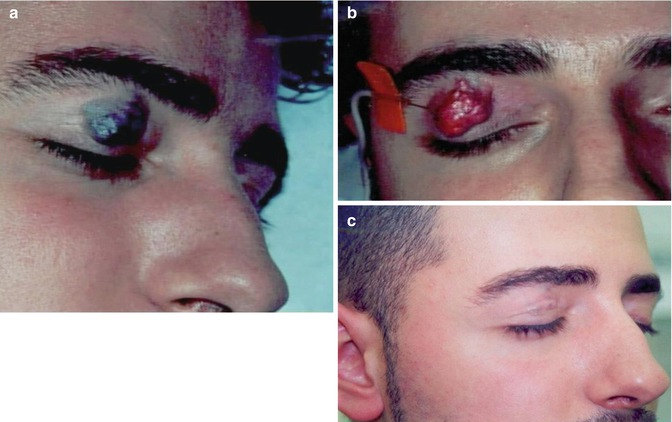
Fig. 34.2
Limited venous malformation of the right upper eyelid. (a) Before, (b) during, and (c) 3 months after sclerotherapy with microfoam
Sclerosing Agents
The sclerosing agents used currently in the treatment in sclerotherapy include sodium morrhuate, sodium tetradecyl sulfate, polidocanol, ethanolamine oleate, ethanol, hypertonic saline, amidotrizoic acid, bleomycin, dextrose, tetracyclines, and OK-432. The selection of the specific agent depends upon the balance efficacy/side effects. In our opinion, the best is polidocanol as a specific kind of patented foam, approved in the USA as Varithena® [7–9].
Sclerotherapy with ethanol, the most widely used agent in the treatment of these lesions, is highly aggressive and associated with major complications due to the lack of control over the injected liquid. In fact, adverse effects have been reported including necrosis of skin and mucosa, deep venous thrombosis in extremities, pulmonary embolism, sensory and motor neurologic lesions, superficial cellulitis, and cardiorespiratory collapse due to bronchial spasm [7]. Moreover, ethanol treatment is not readily repeatable, and this is essential for sclerotherapy, since partial recanalization after the intravascular thrombosis is frequent. Finally, sclerotherapy with ethanol is contraindicated in children and high-risk areas such as eyelids, mucosa, genital area, etc. [10].
Technique of Classic Sclerotherapy
The technique of sclerotherapy must ensure that a precise dose is administered and that the agent is homogeneously distributed on the entire endothelial perimeter of the treated venous segment [9]. It must also ensure that the intravascular concentration of the sclerosing agent is not reduced by dilution in the blood or that this dilution is minimal and always known. Finally, the sclerosant must remain in contact with the endothelium for the necessary time period. In short, the requirements for a successful sclerotherapy are the following:
Knowledge of the intravascular concentration of the sclerosant. |
Homogeneous distribution within the vessel. |
Control over the time of contact between agent and endothelia. |
Sclerotherapy must be selective, i.e., limited to the targeted venous area. |
These requirements are not met by conventional liquid sclerosants. By using these, beside their less safety, the correct dosage on the endothelia and therefore the sclerosing action are highly difficult to control. In addition, in vessels exceeding a certain volume or flow, the therapeutic effect is generally confined to a short segment.
Ethanol sclerotherapy, given its local and systemic secondary effects, is mostly indicated before surgery as a preoperative support to reduce the size of the lesion or as a postoperative complement [4, 8]. In contrast, conventional sclerotherapy of large malformations is ineffective because of the intrinsic limitations of the injected liquids including the following:
Dilution and progressive deactivation of the liquid in a large blood volume |
Irregular distribution of the sclerosant on the endothelia of the treated area |
Difficulties in manipulating and controlling the injected sclerosant |
Imperceptibility of the liquid sclerosant in the vessel by duplex ultrasonography |
The general complications of this technique vary according to the agent and the dose used. They include allergic reactions (especially with sodium tetradecyl sulfate), brain intoxication (especially with ethanol, which must never exceed a maximum volume of 1.2 ml/kg in patients of about 70 kg) [7], hemoglobinuria with possible renal damage, cutaneous necrosis by extravasations or reflux [11], and neurapraxia due to extravascular injection near a motor or sensory nerve.
Sclerotherapy with Microfoam
The introduction of sclerosants in the specific pharmaceutical form of microfoam has transformed the scenario depicted above. Microfoam physically displaces the blood contained in the vessels and minimizes dilution, allowing better knowledge of the intravenous concentration. Micronization of the injected sclerosant dramatically reduces the diameter of the bubbles and thus exponentially increases its surface area, enhancing its therapeutic action and allowing a major reduction in the total dose injected. Moreover, microfoam facilitates a more homogenous distribution of the sclerosant on the endothelial surface and prolongs sclerosant-endothelium contact time. The echogenicity of the microbubbles makes them indirectly visible and the easy manipulability of the injected microfoam allows it to be steered to areas distant from the injection site. In this way, microfoam allows the intravascular administration of a more precise dose of sclerosant [12–15].
Apart from its safety, a further benefit of the sclerosing microfoam is its manageability. The high internal cohesion of microfoam means that it can be aspirated and reinjected after its initial injection. The color of the aspirated microfoam, visible in the catheter, reveals the degree of dilution or intravascular occupation; it ranges from white, when the occupation is exclusive to pink or red, when there is moderate or major dilution, respectively. Furthermore, since microfoam can be steered within the vessels, the filling can be intensified or attenuated according to the sensitivity of the venous area under treatment.
Microfoam is made with 60 % O2 and 40 % CO2 which are physiological gases. A priori injection of a gas into the bloodstream raises alarm about the possibility of a gas embolism. However, CO2 has been intravenously injected without adverse effects at doses of 50–100 cc as a contrast in radiological diagnosis since the 1950s.
Among the advantages of CO2 are its fluidity, allowing the use of fine catheters; the absence of toxicity, even at high doses; and its low cost. Thus, on the one hand, the high solubility of the gases facilitates there metabolism in blood and pulmonary diffusibility, and on the other hand, the micronization increases exponentially the surface of the gas and facilitates its solubility in the corporal liquids. The sclerosant has a greater active surface area for making contact with the endothelium in comparison with the small surface area available to the liquid form. In this way, the dosage of sclerosants in microfoam is more precise and its action more predictable compared with the liquid form. The microfoam does not mix immediately with the blood but displaces it from within the vessel and fills its lumen, so that the sclerosant contacts the endothelia of the veins homogeneously without dilution and at a known intravascular concentration. Because the vessel can be kept full of microfoam, the sclerosant-endothelium contact time can also be controlled at will [13, 14]. Finally, the blood solubility of the injected gases is very important, especially for patients with permeable oval foramen.
Technique of Treatment with Microfoam in Vascular Malformations
First of all, a precise and extensive anatomic and functional evaluation (MRI, CT angiography, and duplex ultrasound) must be performed to define the characteristics of a predominantly venous malformation or of a combined type. Thus, according to Puig et al. [16], the lesions can be divided into four groups:
Limited malformations without peripheral drainage |
Malformations that drain into normal veins |
Malformations that drain into dysplastic veins |
Malformations that drain into ectatic veins |
The technique consists in the injection under ultrasound guidance of the patented polidocanol foam [14]. At each session, 20–100 ml of microfoam is injected, at concentrations ranging between 0.25 and 3 %, in accordance with the location of the malformation and the hemodynamic characteristics of the area to be treated. Thus, infiltrating malformations (Figs. 34.3, 34.4, 34.5, 34.6, 34.7, and 34.8) require higher concentrations (2–3 %), whereas malformed lateral veins such as the marginal vein (Figs. 34.9 and 34.10) are treated with lower concentrations (around 1 %). The procedure is performed without anesthesia and the number of sessions is variable, ranging from a single one to several sessions depending on the size, complexity, and number of the anomalous veins. The interval between sessions is 2 weeks, spacing them to 4 weeks as the treatment advances. Clinical and radiological improvement is usually possible in the great majority of patients (over 90 %).