Salivary Gland Cancer
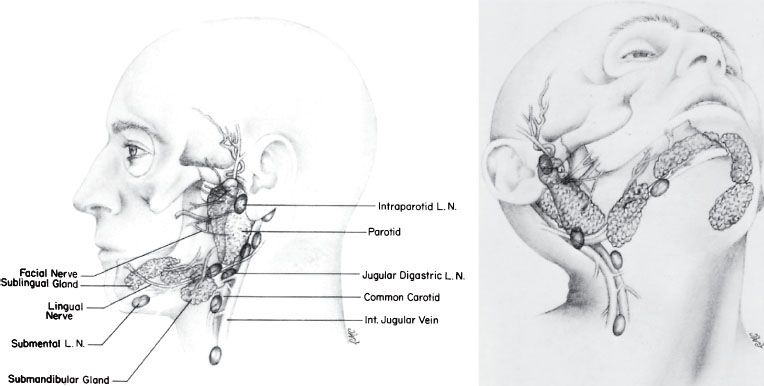
The salivary glands consist of the three large, paired major glands—parotid, submandibular, and sublingual (Fig. 43.1)—and many smaller, minor glands located throughout the upper aerodigestive tract. Salivary gland malignancies make up only approximately 0.4% of all cancers and account for <5% of the annual incidence of head and neck malignancies in the United States. The international variation in the incidence is between 0.4 and 2.6/100,000 per year, with a mean of approximately 1.2/100,000.1,2 No significant change in incidence has been shown in recent decades.1–3
FIGURE 43.1. Anatomy of salivary glands.
 ANATOMY
ANATOMY
Major Salivary Glands
Parotid Gland
The parotid gland is located superficial to and partly behind the ramus of the mandible and covers the masseter muscle. Superficially, it overlaps the posterior part of the muscle and largely fills the space between the ramus of the mandible and the anterior border of the sternocleidomastoid muscle. One or more isthmi that wrap around the branches of the facial nerve connect the superficial and deep lobes of the gland. The nerve enters the deep surface of the gland as a single trunk, passing posterolateral to the styloid process. It usually leaves the gland as five or more branches, emerging at the anterior, upper, and lower borders of the gland. The facial nerve runs superficial to the main blood vessels that traverse the gland but is interwoven within the glandular tissue and its ducts. Thus, removal of all or part of the parotid gland demands meticulous dissection if the nerve is to be spared.
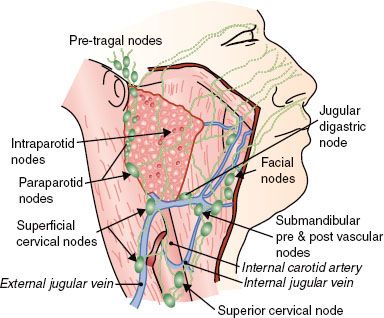
The parotid gland contains an extensive lymphatic capillary plexus, many aggregates of lymphocytic cells, and numerous intraglandular lymph nodes in the superficial lobe. Lymphatics drain from more lateral areas on the face, including parts of the eyelids, diagonally downward and posterior toward the parotid gland, as do the lymphatics from the frontal region of the scalp. Associated with the gland, both superficially and more deeply, are parotid nodes. These drain downward along the retromandibular vein to empty in part into the superficial lymphatics and nodes along the outer surface of the sternocleidomastoid muscle and in part into upper nodes of the deep cervical chain. Lymphatics from the parietal region of the scalp drain partly to the parotid nodes in front of the ear and partly to the retroauricular nodes in back of the ear, which, in turn, drain into upper deep cervical nodes.4
Submandibular Gland
The submandibular gland largely fills the triangle between the two bellies of the digastric and the lower border of the mandible and extends upward deep to the mandible. It lies partly on the lower surface of the mylohyoid and partly behind the muscle against the lateral surface of the muscle of the tongue, the hypoglossus. The submandibular gland has a larger superficial part, or body, and a smaller deep process. The inferior surface is adjacent to the submandibular lymph nodes, and the deep process of the submandibular gland lies between the mylohyoid laterally and the hyoglossus medially and between the lingual nerve above and the hypoglossal nerve below.5 Bimanual palpation with one finger in the floor of the mouth and one under the edge of the mandible facilitates clinical detection of masses in this gland.
A rich lymphatic capillary network lies in the interstitial spaces of the gland (Fig. 43.2). From the lateral and superior portions of the gland, lymph flows to the prevascular or preglandular submandibular lymph nodes. The posterior portion of the gland gives rise to one or two lymphatic trunks, which follow the facial artery and go directly to the anterior subdigastric nodes of the internal jugular chain.6 The nodes overlying the submandibular gland, followed by the subdigastric and high midjugular lymph nodes, are those involved in nodal metastases.
Sublingual Gland
This smallest of the three major salivary glands, along with many minor salivary glands, lies between the mucous membrane of the floor of the mouth above and the mylohyoid muscle below, the mandible laterally, and the genioglossus muscles of the tongue medially. This is a rare site for malignant neoplasms; they are difficult to distinguish from cancer of the floor of the mouth, accounting for <2% of all reported cases of salivary gland tumors.1,7 The sublingual gland drains either to the submandibular lymph nodes or more posterior into the deep internal jugular chain between the digastric and omohyoid muscles. Rarely, the lymphatics of the sublingual gland drain into a submental node or supraomohyoid jugular node.
Minor Salivary Glands
Minor salivary glands are widely distributed in the upper aerodigestive tract, palate, buccal mucosa, base of tongue, pharynx, trachea, cheek, lip, gingiva, floor of mouth, tonsil, paranasal sinuses, nasal cavity, and nasopharynx.
FIGURE 43.2. Lymph node distribution in and around the parotid gland.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Seventy percent of all salivary tumors arise in the parotid gland, 8% in the submandibular gland, and 22% in the minor salivary glands.7 The proportion of malignant tumors increases from parotid (25%) to submandibular (43%) to minor salivary glands (65%).7,8 There is a preponderance of benign tumors in women; malignant tumors exhibit an equal sex distribution.2 Patients with benign tumors are younger (mean age, 46 years) compared with those with malignant tumors (mean age, 54 years), with a trend to an older age for submandibular and minor salivary gland locations.7 From 2% to 3% of salivary neoplasms occur in children, in whom half of the tumors are malignant.9 The majority of cancers are located in the parotid gland, with mucoepidermoid cancers predominating. The tumors in children are mostly less advanced, and a 95% 5-year survival is reached.9
Etiologic factors are not clearly defined. Nutrition may be a factor because Eskimos in the Arctic, who have low intake of vitamins A and C, have a high incidence. Cigarette smoking and alcohol consumption are in general not related to salivary gland cancer,10 although cigarette consumption of >80 pack-years may contribute to salivary gland cancer.11 Irradiation can also be a cause, as evidenced by the increased incidence in survivors of the atomic bombs dropped on Hiroshima and Nagasaki and in those irradiated to the head and neck for benign conditions during childhood.12–15 Saku et al.13 studied salivary gland tumors in atomic bomb survivors of Hiroshima and Nagasaki. Two-thirds of all cases were parotid, and the remainder were equally distributed between submandibular and minor salivary glands. Mucoepidermoid cancer and Warthin’s tumor (benign) were particularly elevated compared with nonexposed persons and disproportionately high at high radiation doses. Modan et al.12 found a clear dose–response effect in a matched control study of patients who had low-dose head–neck irradiation in childhood; there was a 2.6-fold increase of benign tumors and a 4.5-fold increase of cancer. The majority of these tumors are mucoepidermoid cancers14,15; however, <1% of salivary gland tumors may be caused by former irradiation.16
Workers in various occupations experience an increased risk of salivary gland cancer.17 For women employed as hairdressers or working in beauty shops, a significant elevated risk was observed in a study by Swanson and Burns.11
Women with salivary gland cancer may have a 2.5-fold elevated breast cancer risk.18 After treatment for salivary gland cancer, there is an increased risk for subsequent cancer of the oral cavity (hazard ratio [HR] = 3.5) and thyroid (HR = 2.7), lung and kidney cancer (HR = 1.7), and second salivary gland cancer (HR = 10), especially after acinic cell cancer (HR = 31). For adenoid cystic cancer the risk of developing nasopharyngeal is increased by a factor 17.19
 NATURAL HISTORY
NATURAL HISTORY
Local invasion is the initial route of spread of malignant tumors of the salivary glands, depending on location and histologic type. For parotid tumors, this may result in fixation to structures in around 20% of cases.20 Skin invasion is more often seen in parotid tumors (8%–10%) compared with submandibular tumors (3%).21,22
Approximately 25% of patients with a malignant parotid salivary gland tumor present with facial palsy from cranial nerve invasion.7,20,22,23,24
A detailed study by the Dutch Head and Neck Oncology Group (NWHHT) concerning patients with a salivary gland malignancy found an overall incidence of clinically positive nodes of 14% and clinically occult, pathologically positive nodes in an additional 11% of patients.21 This percentage depends on the number of neck dissections performed, the tumor location, histology, and T stage. The number of elective neck dissections performed varies among the tumor locations. Stennert et al.25 performed a neck dissection in all malignant parotid tumors and found 53% unilateral positive nodes and 0% contralateral nodes. In selected patients in other studies, the percentage positive nodes varied between 20%26 and 38%.24 Three of four involved lymph nodes in early parotid malignancies (21% occult nodes) were localized in intraparotideal nodes by Stenner et al.27 Resection of submandibular tumors is combined with a (partial) neck dissection in most cases. Pathologic neck nodes may be seen in up to 42% of cases.24 The risk of positive lymph nodes in minor salivary gland cancer depended on four prognostic factors; male sex, T3-T4, pharyngeal site, and histology (high-grade mucoepidermoid and high-grade adenocarcinoma). Based on these factors, a scoring system from 0 to 4 was developed by Lloyd et al.28 The risk of positive nodes in minor salivary gland cancer is <10% for score 0 to 1, 17% for score 2, 41% for score 3, and 70% for score 4. Salivary gland tumors arising in the oral cavity produce an incidence of cervical node metastases of <10%.8,24,29,30 Nasopharyngeal salivary gland tumors have a high risk of occult metastases (50%).30 In general, the risk of positive findings in the neck, including all salivary gland malignancies, may be based on a combination of T stage, tumor localization, and histology. The highest risk is seen for squamous cell, undifferentiated, and salivary duct cancers.24,31 There is an intermediate risk for mucoepidermoid cancer and a low risk for acinic cell, adenoid cystic carcinoma, and carcinoma ex pleomorphic adenoma.24 A 15% risk is found for T1 tumors, 26% for T2, and 33% for T3–4.24 An example of a rating scale to estimate the risk of positive neck nodes based on tumor location, T stage, and histologic type is shown in Table 43.1.
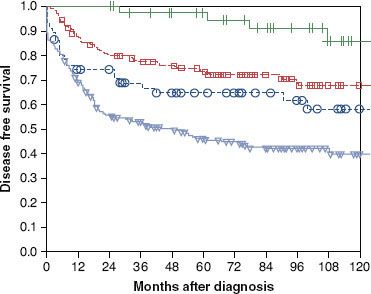
Distant metastases overall are encountered in 3% of patients at presentation and in 33% after 10 years.21 They are fairly common with adenoid cystic, salivary duct, squamous cell, and undifferentiated carcinomas; in the case of adenoid cystic carcinomas, they may occur quite late in the course of the disease, without recurrence of the primary tumor.21,32,33 Distant metastases are primarily to lung, bone, and occasionally liver.21 Reported incidence of distant metastases in patients with adenoid cystic carcinoma after 10 years of follow-up is approximately 40%.21,33,34 Five years after diagnosis of distant metastases of adenoid cystic carcinoma and acinic cell cancer, more than one-third of the patients are still alive; 10% are alive after 10 years. An update of the survival data of the NWHHT study after diagnosis of distant metastases of salivary gland cancer is shown in Figure 43.3.
TABLE 43.1 RISK ESTIMATION (%) FOR POSITIVE NECK NODES
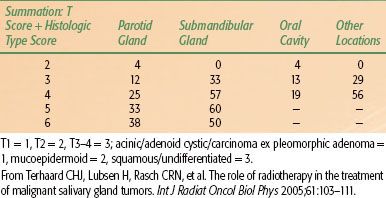
TABLE 43.2 DISTRIBUTION OF PRESENTING SITES OF INTRAORAL MINOR SALVARY GLAND TUMORS
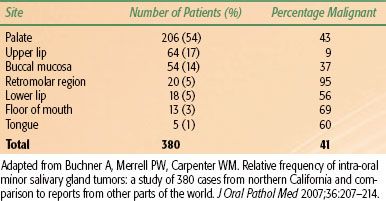
FIGURE 43.3. Survival after diagnosis of distant metastases, depending on histology; update of the nationwide Dutch study. (• ) Adenoid cystic carcinoma49; () acinic cell carcinoma11; () others99; p <.001. (From Terhaard CHJ, Lubsen H, Van der Tweel, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: Results of the Dutch Head and Neck Oncology Cooperative Group. Head Neck 2004;26(8):681–693.)
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Three of four parotid masses are benign.7 Patients most often have a painless, rapidly enlarging mass, often present for years before a sudden change in its indolent growth pattern prompts the patient to seek medical attention. Duration of clinical symptoms before diagnosis may last >10 years.7,21 For malignant tumors, the median duration of clinical symptoms generally is shorter (3 to 6 months)21,35 than that of benign tumors, although for some minor salivary gland tumors, median periods of 2 years have been reported.29,36
Pain is more frequently associated with malignant disease.7 Although as many as one-third of parotid cancers may have facial nerve involvement, only 10% to 20% of patients complain of pain.7,20,21 Pain may appear with involvement of deeper structures (masseter, temporal, and pterygoid muscles). Rarely, tumors of the parotid may involve the base of the skull and cause intractable pain and paralysis of various cranial nerves. Asymptomatic swelling of the floor of the mouth is the primary presentation in sublingual salivary gland cancer, with pain and tongue numbness as symptoms of advanced disease.37
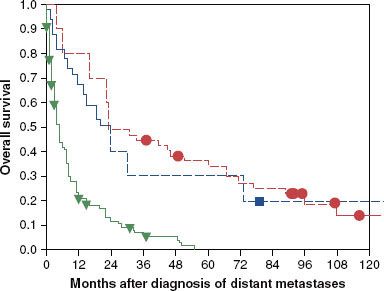
The signs and symptoms associated with tumors of the minor salivary glands vary because of their diverse locations. The distribution of presenting sites and risk of malignancy for 380 cases of intraoral minor salivary gland tumors are shown in Table 43.2.38 A painless lump is the most common presenting symptom. Almost all extraoral minor salivary gland tumors are malignant.7 Extraoral minor salivary gland tumors are most frequently seen in the nasal cavity (52%), followed by the larynx (18%), the oropharynx (14%, mostly localized in the tonsil), nasopharynx (8%), and ethmoid (8%).7 For tumors arising in the nasal cavity or sinuses, facial pain is the most common presenting symptom, followed by nasal obstruction. Laryngeal primary tumors most frequently cause hoarseness or voice change.
Clinical features suggesting a malignant salivary gland tumor are rapid growth rate, pain, facial nerve palsy, childhood occurrence, skin involvement, and cervical adenopathy.
 DIAGNOSTIC WORKUP AND STAGING
DIAGNOSTIC WORKUP AND STAGING
Major Salivary Glands
The diagnostic workup of major salivary gland tumors includes a careful history and physical examination, with particular attention to signs of local fixation or regional adenopathy.
For superficial lesions of the parotid gland, the submandibular gland, and the sublingual gland and evaluation of the cervical lymph nodes, ultrasound (US) is the first diagnostic step, combined with fine-needle aspiration cytology (FNAC). In US, signs of malignancy are ill-defined borders with heterogonous architecture, internal necrosis, and cystic changes.39 Benign salivary tumors are well defined and hypoechoic. FNAC is very useful for differentiating between yes and no neoplastic lesions, with a specificity of >95%. The sensitivity for malignancy may vary according to country, with a mean of around 80%.40 In a systematic review Colella et al.41 found concordant cytology in 80%, 96%, and 94%, respectively, for patients with a histologic diagnosis of a malignant tumor, a benign tumor, or a nonneoplastic lesion. False-negative findings may be seen as result of lack of representative material or a cyst. The relative low negative predictive value of fine-needle aspiration will be improved if magnetic resonance imaging (MRI) and fine-needle aspiration are combined.
The next imaging tool in salivary gland tumors suspected for malignancy is MRI. MRI is superior to computed tomography (CT), especially when malignancy is suspected, based on the excellent soft-tissue contrast (Fig. 43.4). T1-weighted (W) images are excellent to assess the margins, extension into the deep tissues, and patterns of infiltration because the (fatty) background of the gland is hyperintensive. With T1-W gadolinium series, tumor infiltration and heterogeneous enhancement may be visualized, as seen in high-grade tumors.39 With fat-suppressed T2-W images the fluid content of a tumor is visualized. In general, benign tumors are hyperintensive, and malignant tumors show intermediate or low intensity at T2-W MR images. Early contrast enhancement and slow washout are signs of malignancy on dynamic MRI.42 A low apparent diffusion coefficient (ADC), derived from diffusion-weighted MRI using high b values, is associated with malignancy.43 However, Warthin’s tumors (benign) may also show low ADC values.44 Perineural invasion of adenoid cystic carcinoma may be evaluated with both CT (foraminal enlargement) and MRI (thickened nerve with enhancement on the fat-suppressed T1-weighted images).39 The tumor extension along the facial nerve (VII; foramen styloideum), the cranial nerve V-3 (foramen ovale), or nerve V-2 (foramen rotundum), as seen in tumors of the deep parotid lobe, may be visualized by T1-weighted images.43 This is of special importance for the delineation of these nerves in case of postoperative radiotherapy for extensive perineural invasion; see Figure 43.5 for the trigeminal and facial nerves. Cortical involvement is best evaluated by CT.
Fluorodeoxyglucose (FDG) uptake in salivary glands is quite unpredictable, resulting in a low sensitivity of FDG-positron emission tomography (PET) for the diagnosis of malignancy in salivary glands. However, the combination of FDG-PET with CT imaging was a deciding image modality in 15% of cases in a study of Razfar et al.45 The recently developed combined FDG-PET/MRI scans could be a valuable extension of the diagnostic imaging modalities in salivary gland cancer.
The seventh edition of the manual of the American Joint Committee on Cancer and the seventh edition of the classification system of the International Union Against Cancer are identical for major salivary glands (Table 43.3).46 They are based on size, extension, and nodal involvement. Relative survival rates for major salivary gland cancer according to stage are shown in Figure 43.6.
TABLE 43.3 INTERNATIONAL UNION AGAINST CANCER STAGING SYSTEM FOR MAJOR SALIVARY GLAND CANCER (PAROTID, SUBMANDIBULAR, SUBLINGUAL)
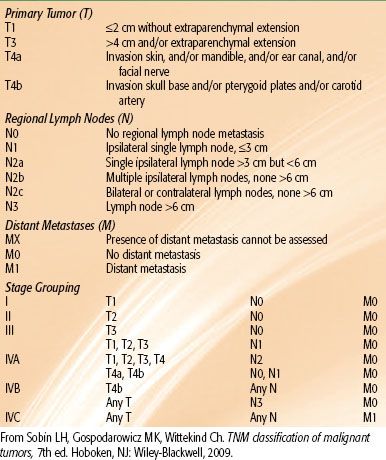
FIGURE 43.4. A patient with acinic cell cancer of the left parotid gland. A: Axial contrast-enhanced computed tomography image. B: T1-weighted magnetic resonance image. Both images show an infiltrating soft tissue mass involving the deep and superficial lobe. The tumor has widened the left stylomandibular tunnel, with infiltration in the pterygoid musculature. The left internal carotid artery is displaced medially. (Courtesy of Dr. F. A. Pameijer, radiologist, University Medical Center, Utrecht, Netherlands.)
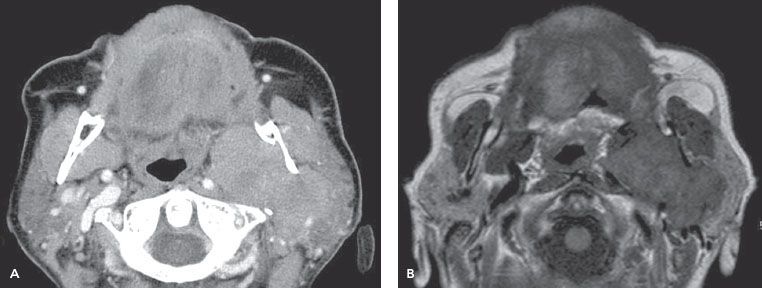
FIGURE 43.5. Three-dimensional magnetic resonance imaging, T2 fast field echo with binomial radiofrequency pulses for water-selective excitation. A: Nerve V. B: Nerve VII.
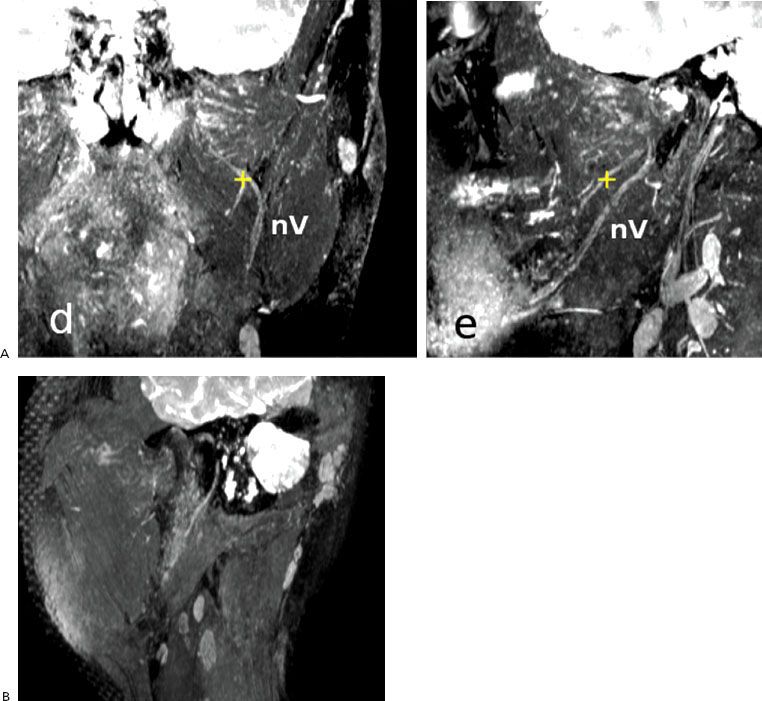
FIGURE 43.6. Disease-free survival for major salivary glands according to the 2002 classification of the American Joint Committee on Cancer. Results of the nationwide Dutch study: +, stage I;  , stage II; , stage III; ∇, stage IV.
, stage II; , stage III; ∇, stage IV.
Minor Salivary Glands
Various radiographic studies may be used, including plain films, to ascertain bone erosion in advanced lesions. CT and MRI scans may be used to evaluate depth, contiguous involvement, and the retropharyngeal nodes. US and FNAC may be used to examine the neck nodes. The definitive diagnostic procedure is an excisional biopsy, particularly if malignancy is clinically expected. Unplanned incisional biopsies should be avoided, and fine-needle biopsies are impractical because of the polymorphism of most malignant salivary gland tumors.
A formal staging system has not been developed for minor gland tumors. The same staging system for minor salivary glands as for squamous cell carcinoma in sites other than the parotid or submandibular glands may be used. The American Joint Committee on Cancer and International Union Against Cancer classification and stage regrouping system has been reported to be a major long-term outcome predictor in minor salivary gland carcinoma.47
TABLE 43.4 WORLD HEALTH ORGANIZATION 2005 CLASSIFICATION OF TUMORS OF THE SALIVARY

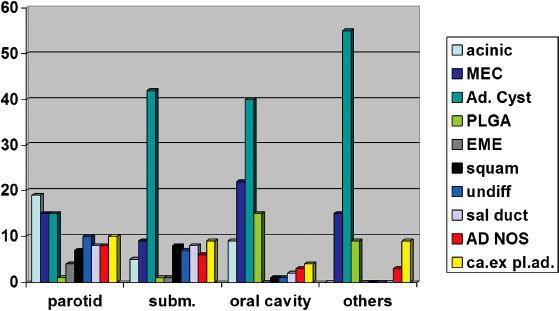
FIGURE 43.7. Distribution of cell types (%) in various series from the Dutch study, depending on site, AD NOS, not otherwise specified. Ad. Cyst, adenoid cystic; ca.ex pl. ad., carcinoma ex pleomorphic adenoma; subm, submandibular; EME, epithelial–myoepithelial carcinoma; MEC, mucoepidermoid cancer; PLGA, polymorphous low-grade adenocarcinoma; sal duct, salivary duct carcinoma; squam, squamous cell carcinoma; undiff, undifferentiated.
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Salivary glands are composed of acinar–ductal units, with acinar cells on the inside of the acinic, and ductal epithelial cells on the inside of the striated and excretory ducts. Myoepithelial cells are located in the outside of acini and intercalated and striated ducts. Basal (reserve) cells are found on the outside of the excretory ducts.48 Carcinomas may arise from all these cell types, separately or combined. The histologic classification of these salivary gland tumors is very demanding for the head and neck pathologist. In 1991 the World Health Organization classification for salivary gland tumors of 1972 was expanded from 7 to 20 subtypes. Various types of carcinomas were distinguished based on precise histologic definitions, prognosis, and treatment (discussed more in detail by Seifert and Sobin49). In 2005 the WHO classification of malignant salivary gland tumors was extended again; 24 subtypes were specified (Table 43.4).48 Classification may be difficult, as shown in a re-evaluation of 101 intraoral salivary gland tumors by experienced pathologists; major disagreement was seen in 8, and there was minor disagreement in 33.50
Most salivary gland subtypes are very rare. The percentage of the histologic subtypes varies from series to series and according to the localization of the tumor (Fig. 43.7). In 666 patients with a salivary gland carcinoma in a study performed in the Netherlands, for which the pathology was revised, adenoid cystic carcinoma was most frequently diagnosed (27%), followed by mucoepidermoid carcinoma (16%), acinic cell carcinoma (14%), carcinoma ex pleomorphic adenoma (8%), undifferentiated carcinoma (7%), salivary duct carcinoma and adenocarcinoma not otherwise specified (both 6%), polymorph low-grade adenocarcinoma (PLGA) and squamous cell carcinoma (both 5%), and epithelial–myoepithelial carcinoma in 2%. In this large study, all other subtypes were rarely or not at all diagnosed.
Salivary gland carcinomas may be graded as of low and high malignancy, particularly for mucoepidermoid tumors. However, there is a disparity in grading, even among experienced pathologists.51 Low-grade mucoepidermoid, PLGA, epithelial–myoepithelial, and acinic cell carcinomas comprise a group of low to moderate malignancy; high-grade mucoepidermoid, malignant mixed, adenoid cystic, squamous, undifferentiated, and salivary duct carcinomas represent higher-grade malignancies.52
Adenoid cystic carcinoma is most common in minor salivary glands,8,21,30,47 followed by the submandibular gland.21,53,54,55 Perineural invasion is common in adenoid cystic carcinoma.56 The adenoid cystic variety has a tubular pattern that has been associated with the best prognosis, a cribriform pattern with an intermediate prognosis, and a solid pattern with the worst prognosis.57
In parotid tumors in children and adults, the most common malignant subtype is mucoepidermoid carcinoma (MEC).9,55,58–60 MEC contains squamous cells, mucus-producing cells, and cells of intermediate type. Several grading criteria and a cell cycle–based Ki-67 proliferation index for MEC have been published.48 Acinic cell cancer derives from cells of the terminal ducts and intercalated ducts. Grading for acinic cell cancer is controversial.61 Most tumors (86%) are located in the parotid gland.61 As in MEC, the Ki-67 proliferation index is a prognostic factor for disease-free survival.48 Carcinoma ex pleomorphic adenoma may be localized in all salivary glands, although the majority is found in the parotid gland. In the development of carcinoma ex pleomorphic adenoma a progressive loss of heterozygosity of chromosome 8q, 12 G, and 17P is seen.62 The prognosis is poor, with a high risk of distant metastases.21 Undifferentiated and squamous cell carcinomas are mainly localized in the parotid gland. The prevalence of comorbidity for patients with squamous cell carcinoma of the salivary gland is comparable to that of other head and neck cancers, whereas the prevalence of comorbidity in all other malignant salivary gland cancer subtypes is significantly less.63 In addition, patients with squamous cell carcinoma of the salivary glands are mostly older.2 Thus, squamous cell carcinoma of the salivary gland may be a different entity among salivary gland cancers. PLGA, salivary duct, and epithelial–myoepithelial carcinoma are the most common new subtypes of the World Health Organization 1991 classification. PLGA is a solid, ovoid, noncapsulated mass with a highly variable growth pattern (Fig. 43.8A). Most are located in the palate, and the prognosis generally is good, with a tendency to local and sometimes regional recurrences.64,65 No distant metastases were seen in 34 patients with PLGA in the retrospective Dutch study. Salivary duct carcinoma resembles ductal breast cancer morphologically (Fig. 43.8B). They derive from excretory duct cells. They are usually located in the parotid gland and are highly aggressive.32,66 They are frequently positive for androgen receptors and positive for HER-2/neu protein. However, gene amplification is seen in <50% of cases.48 There is also a low-grade subtype, resembling breast atypical ductal hyperplasia and low-grade ductal carcinoma in situ. The prognosis is excellent.67 Epithelial–myoepithelial carcinoma is mainly localized in the parotid gland. The tumors are composed of myoepithelial cells surrounded by epithelial-lined ducts resembling intercalated ducts. It is a low-grade tumor; all 14 patients of the Dutch study remained disease free. However, local recurrences may be encountered.68 Myoepithelial carcinomas have exclusive myoepithelial differentiation. They are commonly seen in the parotid gland and the palate. Differentiation with other salivary glands cancers may be difficult. The prognosis is poor, with a 40% 5-year disease-free survival.69 Basal cell adenocarcinoma is a low-grade tumor, predominantly seen in the parotid gland, with a high risk of local recurrence but a low risk of regional recurrences or distant metastases.70 Malignant oncocytoma is a high-grade malignant tumor, characterized by oncocytes, with necrosis, perineural spread, vascular invasion, and a high risk of cervical lymph nodes.71 Most tumors are found in the parotid or submandibular gland. Salivary gland cystadenocarcinoma is a low-grade tumor; two of three are localized in the parotid gland, with others in the oral cavity.72 Mucinous adenocarcinoma is a high-grade malignancy of the palate and floor of the mouth, with a high risk for neck node and distant metastases.73 Sebaceous carcinoma is mainly localized in the parotid gland and is a slow-growing, low-grade tumor.74
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
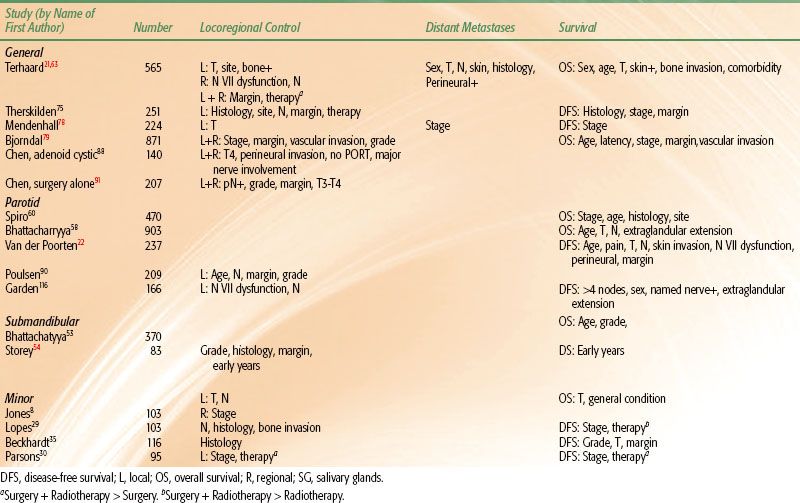
A number of prognostic variables have been studied in the management of salivary gland cancer. In these studies, multivariate analyses have been performed considering locoregional control, distant metastases, and survival. Results of studies with a sufficient number of patients and follow-up are summarized in Table 43.5. Local control and overall survival are influenced by site, favoring tumors of the oral cavity.21,75 T and N stages are independent variables for locoregional control, distant metastases, and survival, regardless of site.8,21,34,52,58,59,75–77,78–79 As can be concluded from Table 43.5, in almost all studies histologic subtype is not an independent prognostic factor for locoregional control, but it plays a role in risk for distant metastases and overall survival.21,75 The added prognostic value of cytologic and/or histologic factors in salivary carcinoma is limited, largely due to the combined prognostic value of other prognostic factors such as tumor size, N and M classification, and comorbidity.80 Comorbidity is associated with overall survival, but not with disease-free survival.63
Oncogene expression has been evaluated in the search for additional prognostic factors. Expression of the oncoprotein p53 was found in an Italian study to be higher in malignant tumors than in benign tumors.81 Furthermore, tumors with moderate-to-high expression of p53 were more frequently associated with regional and distant metastasis and a lower disease-free and overall actuarial survival rate compared with patients with no p53 expression. Univariate and multivariate analyses confirmed the independent prognostic value of p53 expression. Vascular endothelial growth factor significantly correlates with p53 expression and is an independent prognostic factor for survival for salivary gland cancer.82 Overexpression of HER-2/neu was seen in approximately one-third of mucoepidermoid carcinomas in a series of 50 parotid gland cancers83 and in approximately 20% of salivary duct carcinomas.66,84 Overexpression was seen and appeared to be an independent marker of poor prognosis. It also has been associated with poor prognosis in carcinomas of the breast, ovary, and endometrium. Another molecular feature studied in relationship to prognosis is the DNA content in adenoid cystic carcinomas. DNA aneuploidy is correlated with the solid type and thus with poor prognosis.57
FIGURE 43.8. Examples of polymorphous low-grade adenocarcinoma (A) and salivary duct carcinoma (B). (Courtesy of Prof. Dr. P. Slootweg, pathologist, Radboud University Medical Center, Nijmegen, Netherlands.)
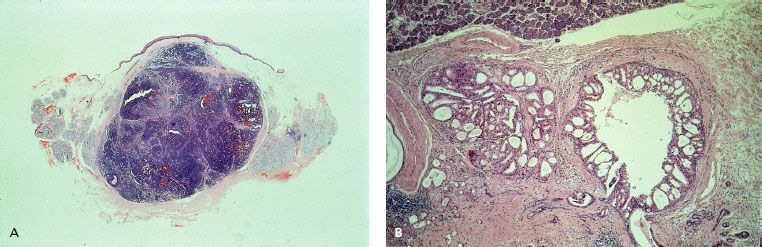
TABLE 43.5 PROGNOSTIC FACTORS FOR SALIVARY GLAND CANCER—SELECTION OF MULTIVARIATE ANALYZED STUDIES
Major Salivary Glands
The survival of patients with submandibular cancers is inferior to that of patients with parotid cancers according to a study by Spiro et al.60 Extraglandular extension58,59 and skin invasion22,52,85 in parotid cancers result in decreased disease-free survival. More-advanced age was found to be a negative prognostic factor for locoregional control in some studies20,31,76 and for disease-free and overall survival in most studies.22,52,58,60,77 Impairment of function of the facial nerve is a known prognostic factor, influencing not only locoregional control,21,59,85 but also disease-free survival.52,77,85,86 Pain at presentation may be associated with reduced disease-free survival.52 Perineural invasion and pain are closely related: in some studies, not pain but perineural growth is an independent prognostic factor for distant metastases21 or disease-free survival.59,87,88
The importance of histologic subtype for major salivary gland cancer varies in published studies. In most studies, histologic types are subdivided into low and high grade. The main prognostic significance of grading relates to disease-free survival,58,79,82 although grade was not a prognostic factor in most studies. The best prognosis is seen for acinic cell and (low-grade) mucoepidermoid cancer,21,60 the worst for undifferentiated21,85 and squamous cell cancer.21,60 At the Netherlands Cancer Institute, a prognostic score for patients with parotid carcinoma was developed and validated.22,52 The preoperative prognostic score is based on a weighted combination of prognostic factors (age, pain, clinical T and N stages, skin invasion, and facial nerve dysfunction); histology and grading are not incorporated. Four subgroups were formed, with markedly different prognoses. In the postoperative score, perineural invasion and positive surgical margins were also included. In adenoid cystic carcinoma, named major nerve involvement is a prognostic factor; however, this may not be true for microscopic invasion only.89 Positive or close surgical margins result in an increase in local recurrence rate.21,54,75,79,90,91 Radiation therapy in addition to surgery improves locoregional control in patients with adverse prognostic factors.20,24,75,85,88,92,93 Improvement of survival has only been shown in two studies53,85 and for stage III and IV major salivary glands in a matched-pair analysis.94
Minor Salivary Glands
The poorest prognosis is associated with adenoid cystic carcinoma.29,35 Stage, base-of-skull involvement, and bone invasion are risk factors for locoregional recurrence and survival in minor salivary gland cancers.8,29,35,90,95 Locoregional control may be improved by adding postoperative radiotherapy.30
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
The general management of salivary gland malignancies in most patients includes surgical excision, followed by radiation therapy for unfavorable prognostic factors (Table 43.5). Postoperative radiotherapy to enhance local control is recommended for T3–4 tumors, close or incomplete resection, bone involvement, perineural invasion, high-grade cancer, and recurrent cancer.21,35,54,59,76,85 Postoperative radiotherapy is indicated for patients with neck node lymph metastases.21,91 Elective neck radiotherapy prevents nodal relapses in a selected group of patients, but in general is not indicated for acinic cell or adenoid cystic carcinoma.96 According to Chen et al.,88,93 for carcinoma ex pleomorphic adenoma of the parotid gland and adenoid cystic carcinoma, surgery and postoperative local radiotherapy is the standard. Concurrent postoperative platinum- and/or paclitaxel-based chemoradiotherapy for high-risk patients (nodal involvement, microscopic positive margins, T3/T4, perineural involvement) may result in 3-year locoregional control of >90%, although in the published data the number of patients is small, the follow-up is short, and the acute toxicity is more severe.97,98 In a small (n = 24) matched control study, postoperative platinum-based chemoradiation for locally advanced major salivary gland cancer improved overall survival significantly compared to postoperative radiation alone; of interest, progression-free survival was only 55% for both groups.99 In general, there is little proof that for salivary gland cancer concurrent postoperative chemoradiotherapy is superior to postoperative radiation alone. For advanced, inoperable, and recurrent salivary gland cancers, primary neutron therapy may lead to superior local control rates compared with primary photon therapy, without evidence of improved survival rates.95,100,101 The use of conventional radiation therapy along with hyperthermia has been reported to have similar efficacy in this patient population.102
Major Salivary Glands
Surgical technique depends on location and extent of primary disease and regional adenopathy. Preservation of the facial nerve, at least partially, followed by postoperative radiotherapy is the preferable treatment unless the facial nerve is involved by tumor.103 Aggressive surgery does not improve disease-free survival. A decrease in extended surgery, resulting in a decrease of sacrifice of the facial nerve, has been shown in the course of years.60 Cable facial nerve grafting with the greater auricular or sural nerve graft decreases the incidence of facial palsy postoperatively, especially if branches and not the main trunk are involved.104 Adjuvant postoperative radiotherapy has no negative effect on facial nerve function.104
Surgical treatment includes neck dissection in cases of clinically positive nodes, followed by postoperative radiotherapy.24 The risk of occult nodal disease depends on T stage and histologic type.24,96 As shown in the scoring system in Table 43.1, the decision to treat the neck for parotid tumors is indicated by a score of at least 4.24 When local prognostic factors indicate postoperative radiotherapy, no elective neck dissection has to be performed; the neck nodes will also be irradiated.25,105 Parotid tumors with facial nerve weakness are associated with frequent occult neck nodes; elective treatment is also indicated.105 The first echelon neck nodes in parotid salivary gland cancer are the intraparotideal,27,106 followed by level II, III, and IV nodes.96,107 Involvement of level I nodes is an independent risk factor for disease-free survival.106 Level I and V nodes are only involved if other levels are positive.107 Contralateral nodes are not at risk. Thus, in case of prophylactic treatment of the clinical N0 neck, at least levels II and III should be incorporated, followed by postoperative radiotherapy in case of pathologic positive nodes of levels I to V. In most cases, elective neck dissection of levels I to III combined with a local resection is performed for submandibular tumors. There is no indication for neck dissection for T1 acinic or T1 adenoid cystic tumors (Table 43.1).24,54,96
Minor Salivary Glands
The treatment of minor salivary gland tumors varies with location but usually involves an attempt at adequate surgical excision first. Irradiation has been used in surgically inaccessible sites or combined with surgery because of locally aggressive tumor behavior and the occurrence of incomplete resection.21,30,35,108 For tumors arising in the palate, tongue, floor of the mouth, oral cavity, or oropharynx, surgical exposure is readily available, and resection usually can be accomplished with acceptable morbidity. Tumors arising in the posterior nasal cavity, nasopharynx, or sphenoid region, however, are relatively inaccessible and are mostly treated with radiation therapy.108 The indication of elective neck treatment may depend on a prognostic index (sore 0 to 4) using four clinicopathologic factors associated with increased risk on positive nodes: male sex, stage T3/T4, pharyngeal location, and high grade.28 For a score of ≥2, elective treatment of the neck nodes is indicated. Surgery alone may be used to treat early-stage hard-palate lesions without evidence of positive margins, perineural spread, or bone invasion; simple excision must be avoided.35 Patients with adenoid cystic carcinoma can have a long natural history with late recurrences,108 and consideration should be given to careful surgical reconstruction and rehabilitation because even patients who are not cured can live many years before dying of disease (see Fig. 43.3).21 Occasionally, a patient may present after simple excision (shelling out) of a lesion, and the pathologic examination shows adenoid cystic carcinoma. If re-excision would cause significant functional or cosmetic sequelae, irradiation alone may be used.30 However, simple excision is not recommended as the initial management of these tumors because of the potential for a significant volume of residual disease.
 RADIATION THERAPY TECHNIQUES
RADIATION THERAPY TECHNIQUES
Pleomorphic Adenoma
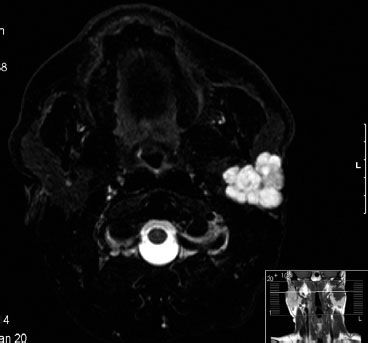
Pleomorphic adenoma (benign mixed tumor) is histologically benign, occurs frequently in a relatively young population, and comprises 65% to 75% of all parotid epithelial tumors.60 Standard therapy has been conservative (superficial) parotidectomy, with recurrence rates of about 0% to 5%.109 Simple excision results in a high recurrence rate of around 25%, as focal capsular exposure occurs in virtually all cases.109 In the past at some institutions, local excision and radiation therapy were used to lower the frequency of facial nerve injury and Frey’s syndrome.110 Dawson and Orr110 reported results for 311 patients. They found a 2.5% recurrence rate at 10 years and an additional 5.5% by 20 years. None of the patients had malignant recurrences at 10 years, 0.5% had such recurrences at 15 years, and 3% had recurrences at 20 years. The later recurrences were more likely to show malignant transformation. The authors concluded that the primary treatment should be surgery because of the patient’s young age, the benign histology, and the remote possibility of subsequent radiation-induced malignancy. However, certain patients may be referred for radiation therapy.111 In a study by Riad et al.112 tumor size of >4 cm, adherence to the facial nerve, and close safety margins, were confounding factors for recurrence, however, because were they correlated with tumor puncture and spillage. Indications for postoperative irradiation may include recurrent disease; microscopically positive margins after surgical resection; and large, deep-seated lesions that may not allow complete surgical excision with adequate margins or would require sacrificing the facial nerve.110,111,113,114 Radiotherapy may decrease the risk of a second recurrence in case of multinodular recurrence only but not for uninodular disease.114 The entire parotid area should be irradiated with a dose of 50 to 60 Gy in 5 to 6 weeks. Figure 43.9 shows an example of a large, multicystic pleomorphic adenoma with a close safety margin and spillage during parotidectomy; postoperative radiotherapy was indicated.
Parotid Gland
The volume of irradiation is determined by pathologic findings, such as perineural invasion of a major nerve. Typically, the entire ipsilateral parotid gland is delineated on the postoperative CT scan performed in a custom-made head and neck mold.59,115 The delineation of the clinical target volume is individualized based on the extent of the disease and surgery.59 In case of a tumor of the deep lobe, the parapharyngeal space and the infratemporal fossa have to be covered adequately.115 In general it is not necessary to treat the scar to full skin dose because only 1% of the patients have a scar failure.76 For very superficial localized tumors and in case of skin invasion, a bolus over the scar is required. In tumors with named perineural invasion (e.g., adenoid cystic carcinoma) the nerve provides a route to the base of skull. Thus, it is important to cover the cranial nerve pathways from the parotid up to the base of the skull.89,116 In perineural invasion, postoperative radiotherapy with a dose of 50 Gy, including the base of the skull, decreased local recurrence rate from 15% with surgery alone to 5% with combined surgery and radiotherapy in a study by Chen et al.117 Focal perineural invasion only is not an indication for routine inclusion of the nerve pathways.116 A clear relationship between dose and local control was only found by Chen et al.,88 a dose of ≥60 Gy resulted in significant higher local control rates. In general, a dose of at least 60 Gy postexcision is recommended24,88,115,116 and at least 66 Gy (33 fractions) for high-risk patients with positive margins (<1 mm).59,116
The ipsilateral neck is treated after a neck dissection has been performed for positive nodes; levels I to V should be included.24 There is no indication for bilateral elective neck treatment.25,27,96 The recommended postoperative dose for positive nodes is at least 60 Gy (30 fractions) and is 66 Gy for extranodal disease.24 Elective irradiation of the neck should be considered for advanced T stage, certain histologic subtypes (Table 43.1), facial nerve dysfunction at presentation, and recurrent disease. The intraparotideal nodes should be included.27,106 At least in early high-risk parotid cancer, levels II and III should be included.27 Levels I and V are seldom positive if only one positive node is diagnosed. However, because in elective treatment the number of possible positive nodes is unknown, most authors advise treatment of levels Ib to IV prophylactically.24,105,118 A dose of approximately 46 to 50 Gy is recommended.24,59
Three basic radiation therapy approaches are used, depending on available equipment: conventional, three-dimensional conformal radiation therapy (3DCRT), and intensity-modulated radiation therapy (IMRT). The first involves unilateral anterior and posterior wedged pair fields using 60Co or 4- to 6-MV photons (Fig. 43.10A). Typical field boundaries are the zygomatic arch superiorly, the masseter muscle anteriorly, the pterygoid muscle and ramus mandibulae laterally, the mastoid process posteriorly, and the posterior belly of the digastric muscle inferiorly.93 A slight inferior angulation of the beams avoids an exit dose through the contralateral eye. A simpler technique uses homolateral fields with 12- to 16-MeV electrons in combination with photons.69,119 Usually, 80% of the dose is delivered with electrons and 20% with 60Co or 4- to 6-MV photons to spare the opposite salivary gland, reduce mucositis, and decrease the skin reaction produced by electrons (Fig. 43.10B). Yaparpalvi et al.119 compared nine conventional treatment techniques. Ipsilateral wedge pair technique with 6-MV photons, wedged anteroposterior and posteroanterior and lateral technique with 6-MV photons, and mixed beam using 6-MV photons and 16-MeV electrons (1:4 weighted) were most optimal, considering dose homogeneity within the target and dose to normal tissues. Electron beam (9 to 12 MeV) and tangential photon fields are effective conventional techniques for sparing the underlying spinal cord (from doses >45 Gy) and the opposite parotid gland in elective neck irradiation. Conventional techniques do not allow for tissue heterogeneity (air cavity, dense bones, and tissues); underdose and overdose may be seen. Thus, conformal techniques should be the treatment of choice.
FIGURE 43.9. Multicystic pleomorphic adenoma, T2 short tau inversion recovery, transverse slice.