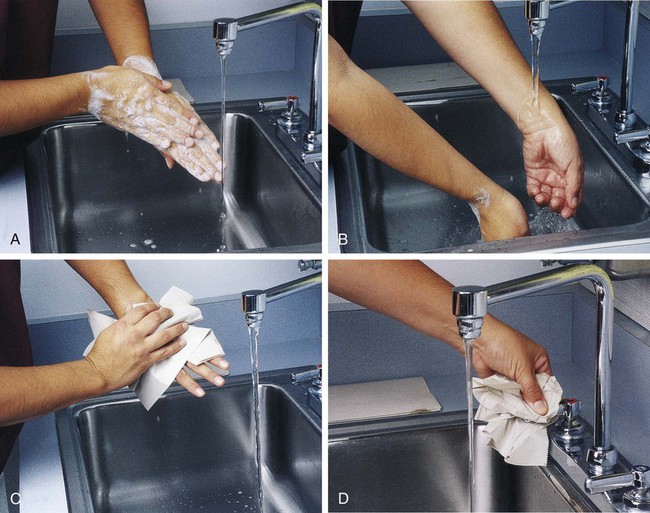
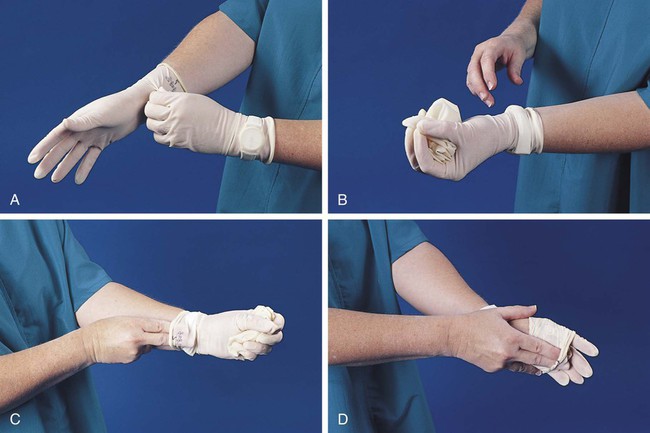
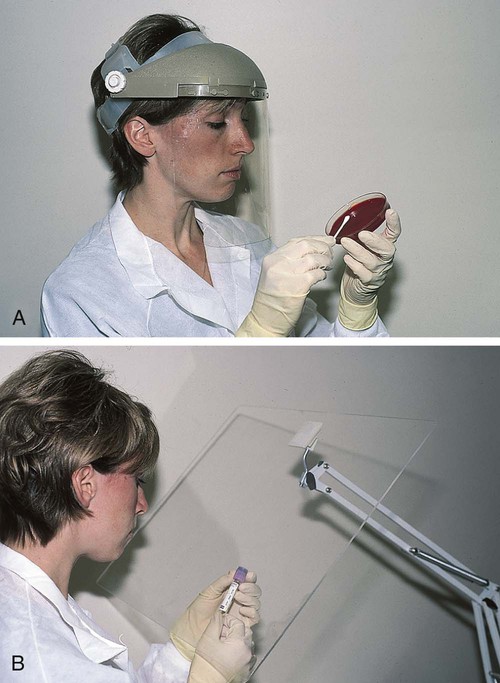
After completion of this chapter, the reader will be able to: 1. Define standard precautions. 2. List infectious materials included in standard precautions. 3. Describe the safe practices required in the Occupational Exposure to Bloodborne Pathogens Standard. 4. Identify occupational hazards that exist in the hematology laboratory. 5. Identify the requirements of the Occupational Exposure to Hazardous Chemicals in Laboratories Standard. 6. Discuss the development of a safety management program. 7. Describe the principles of a fire prevention program, including details such as the frequency of testing equipment. 8. Name the most important practice to prevent the spread of infection. 9. Given a written laboratory scenario, assess it for safety hazards and recommend corrective action. 10. Select the proper class of fire extinguisher for a given type of fire. 11. Define material safety data sheets (MSDSs), list information contained on MSDSs, and determine when MSDSs would be used in laboratory activity. 12. Name the specific practice during which most needle stick injuries occur. 1. A hematology technologist was observed removing gloves and immediately left the laboratory for a meeting. The medical laboratory professional did not remove the laboratory coat. 2. Food was found in the specimen refrigerator. 3. Syringes were found in the proper sharps container. On further investigation, 50% of the attached needles were recapped. 4. Hematology technologists were seen in the lunchroom wearing laboratory coats. 5. Fire extinguishers were found every 75 feet of the laboratory. 6. Fire extinguishers were inspected quarterly and annually. 7. One fire drill was conducted in the last 8 months. 8. Unlabeled bottles were found at the workstation. 9. A 1 : 10 solution of bleach was found near the electronic cell counter. Further investigation revealed that the bleach solution was made 6 months ago. 10. Gloves were worn by the staff receiving specimens. 11. Material safety data sheets were obtained by fax. 12. Chemicals were stored alphabetically. 13. Fifty percent of the staff interviewed had not participated in a fire drill. The following standards are applicable in a hematology laboratory and must be enforced: 1. Handwashing is one of the most important safety practices. Hands must be washed with soap and water. If water is not readily available, alcohol hand gels (minimum 62% alcohol) may be used. Hands must be thoroughly dried. The proper technique for handwashing is as follows: a. Wet hands and wrists thoroughly under running water. b. Apply germicidal soap and rub hands vigorously for at least 15 seconds, including between the fingers and around and over the fingernails (Figure 2-1, A). c. Rinse hands thoroughly under running water in a downward flow from wrist to fingertips (see Figure 2-1, B). d. Dry hands with a paper towel (see Figure 2-1, C). Use the paper towel to turn off the faucet handles (see Figure 2-1, D). a. Whenever there is visible contamination with blood or body fluids c. After gloves are removed and between glove changes d. Before leaving the laboratory e. Before and after eating and drinking, smoking, applying cosmetics or lip balm, changing a contact lens, and using the lavatory f. Before and after all other activities that entail hand contact with mucous membranes, eyes, or breaks in skin 2. Eating, drinking, smoking, and applying cosmetics or lip balm must be prohibited in the laboratory work area. 3. Hands, pens, and other fomites must be kept away from the worker’s mouth and all mucous membranes. 4. Food and drink, including oral medications and tolerance-testing beverages, must not be kept in the same refrigerator as laboratory specimens or reagents or where potentially infectious materials are stored or tested. 5. Mouth pipetting must be prohibited. 6. Needles and other sharp objects contaminated with blood and other potentially infectious materials should not be manipulated in any way. Such manipulation includes resheathing, bending, clipping, or removing the sharp object. Resheathing or recapping is permitted only when there are no other alternatives or when the recapping is required by specific medical procedures. Recapping is permitted by use of a method other than the traditional two-handed procedure. The one-handed method or a resheathing device is often used (see Chapter 3). Documentation in the exposure control plan should identify the specific procedure by which resheathing is permitted. 7. Contaminated sharps (including, but not limited to, needles, blades, pipettes, syringes with needles, and glass slides) must be placed in a puncture-resistant container that is appropriately labeled with the universal biohazard symbol (Figure 2-2) or a red container that adheres to the standard. The container must be leak-proof (Figure 2-3). 8. Procedures such as removing caps when checking for clots, filling hemacytometer chambers, making slides, discarding specimens, making dilutions, and pouring specimens or fluids must be performed so that splashing, spraying, or production of droplets of the specimen being manipulated is prevented. These procedures may be performed behind a barrier, such as a plastic shield, or protective eyewear should be worn (Figure 2-4). 9. Personal protective clothing and equipment must be provided to the worker. The most common forms of personal protective equipment are described in the following: a. Outer coverings, including gowns, laboratory coats, and sleeve protectors, should be worn when there is a chance of splashing or spilling on work clothing. The outer covering must be made of fluid-resistant material, must be long-sleeved, and must remain buttoned at all times. If contamination occurs, the personal protective equipment should be removed immediately and treated as infectious material. b. Gloves must be worn when the potential for contact with blood or body fluids exists (including when removing and handling bagged biohazardous material and when decontaminating bench tops) and when venipuncture or finger sticks are performed. One of the limitations of gloves is that they do not prevent needle sticks or other puncture wounds. Provision of gloves to laboratory workers must accommodate latex allergies. Alternative gloves must be readily accessible to any laboratory worker with a latex allergy. Gloves must be changed after each contact with a patient, when there is visible contamination, and when physical damage occurs. Gloves should not be worn when “clean” devices, such as a copy machine or a “clean” telephone, are used. Gloves must not be worn again or washed. After one glove is removed, the second glove can be removed by sliding the index finger of the ungloved hand between the glove and the hand and slipping the second glove off. This technique prevents contamination of the “clean” hand by the “dirty” second glove (Figure 2-5).1 Contaminated gloves should be disposed of according to applicable federal or state regulations. c. Eyewear, including face shields, goggles, and masks, should be used when there is potential for aerosol mists, splashes, or sprays to mucous membranes (mouth, eyes, or nose). Removing caps from specimen tubes, working at the cell counter, and centrifuging specimens are examples of tasks that could result in creation of aerosol mist. 10. Phlebotomy trays should be appropriately labeled to indicate potentially infectious materials. Specimens should be placed into a secondary container, such as a resealable biohazard-labeled bag. 11. If a pneumatic tube system is used to transport specimens, the specimens should be transported in the appropriate tube (primary containment), placed into a special self-sealing leak-proof bag, appropriately labeled with the biohazard symbol (secondary containment). Requisition forms should be placed outside of the secondary container to prevent contamination if the specimen leaks. Foam inserts for the pneumatic tube system carrier prevent shifting of the sample during transport and also act as a shock absorber, thus decreasing the risk of breakage.
Safety in the Hematology Laboratory
Case Study
Standard Precautions
Applicable Safety Practices Required by the OSHA Standard

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Safety in the Hematology Laboratory