Fig. 7.1
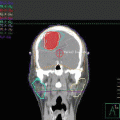
Chest X-ray at presentation showing widening mediastinum
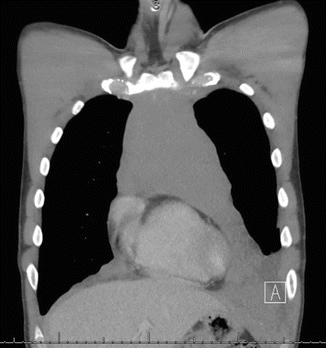
Fig. 7.2
CT chest at presentation showing the disease starting at the left neck to the lower mediastinum with pleural and pericardial effusion
A transthoracic core biopsy was done (Fig. 7.3), and the diagnosis of T–lymphoblastic lymphoma was made. The pathology specimen revealed prominent population of aberrant T cells (75 % of total cells), which are positive for CD1a, surface CD3, CD4, CD7, CD8, and CD45 and negative for CD33.
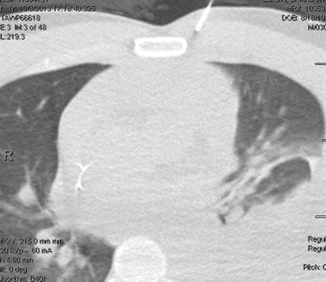

Fig. 7.3
Showing the biopsy taken from the mediastinal mass
The proliferation index is approximately 80–90 % by Ki–67 stain. The rest of the workup included bone marrow biopsy and blood work to rule out blood involvement, and they both came back negative.
Clinical Presentation and Pathology
Lymphoblastic lymphoma (LBL) is mainly a T-cell neoplasm (90 %) [1] that resembles acute lymphoblastic lymphoma (ALL) with no or low risk for bone marrow involvement. It is grouped with ALL in the 2008 WHO classification [2]. The lack of bone marrow involvement (<25 % of blasts) along with a mediastinal mass originating from the thymus forms the unique presentation that makes this disease a distinct entity. Clinically, the hallmark is a mediastinal bulky disease in a young man, and the ratio of male to female is 2:1. Often, the mediastinal mass will grow to a size that will cause acute symptoms that will send the patient to the emergency room. Symptoms might include shortness of breath, chest pain, and superior vena cava syndrome. The mediastinal mass is often associated with a pleuropericardial effusion [3]. According to our institutional data, 70 % of patients present with stage III/IV, 70 % with mediastinal involvement, and only 9 % with CNS disease at presentation. Other groups reported up to 20 % CNS disease especially with no CNS prophylaxis. Other sites of involvement, although <10 %, includes the spleen/liver, kidney, bone, and skin.
Pathologically it is a neoplasm of immature T cells. This is also reflected by different stages of maturations seen. A recent study showed that the level of maturation could actually affect the clinical outcome. Early T-cell precursor (ETP) differentiation stage (recently immigrated cells from the bone marrow to the thymus and with a typical phenotype of CD1a−, CD8−, and CD5− and positivity for myeloid antigens) was associated with a lower remission rate as well as median survival compared to no ETP subtypes including thymic and mature subtypes [4].
For the patient in case 1, after completing workup including assessment of the heart and liver function, he was initiated on treatment as per our institutional guidelines using leukemia–like regimen hyper–CVAD (hyper–cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with high–dose cytosine arabinoside (Ara–C) and methotrexate for a total of 8 cycles given usually over 4–5 months. There was no attempt to drain the pleural effusion. Every attempt was made to control and prevent tumor lysis syndrome. Concomitantly, the patient received central nervous system (CNS) prophylaxis using intrathecal chemotherapy with methotrexate on day 2, and Ara–C on days 7 and 8 of each cycle. An interim CT chest at the completion of 4 cycles showed almost complete disappearance of the mediastinal mass with a bone marrow still showing no evidence of disease. At the completion of chemotherapy and before starting maintenance therapy, the patient was referred for consolidation radiation therapy; Fig. 7.4 shows the status of the mediastinum at completion of his chemotherapy.
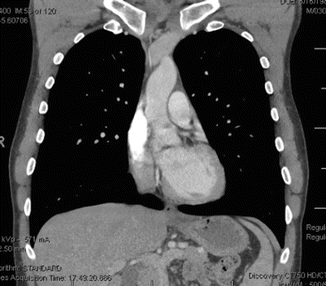

Fig. 7.4
Showing the complete remission at the end of chemotherapy
Treatment of LBL with Chemotherapy
The cornerstone of treatment of LBL is leukemia-like regimens, which differ from one institution to another. The treatment regimens used are based on the successful experience in treating childhood leukemia. The German group regimen NHL-BFM 90 resulted in an outstanding 5-year event-free survival in children of 90 % [5]. Building on the pediatrics experience, the University of Texas MD Anderson Cancer Center reported a good outcome in adult leukemia using hyper-CVAD [6]. The same regimen was used for the treatment of patients with LBL with 91 % achieving complete remission and a 3-year progression-free and overall survival of 66 % and 70 %, respectively. Other investigators adopted different regimens, but it is widely accepted that more intensive regimens are superior, and it is also accepted that adding CNS prophylaxis decreases the risk of CNS relapse that could be as high as 26 % [7]. After completion of induction and consolidation chemotherapy, which in case 1 was 8 cycles of alternating hyper-CVAD and high MTX-Ara-C, patients will start maintenance therapy for 2 years consisting of intravenous POMP (6 mercaptopurine, methotrexate, vincristine, and prednisone).
The patient in case 1, as per our institutional policy, presented to the radiation oncology department to receive consolidation radiation therapy to the mediastinal area at the completion of his last chemotherapy cycle. It was insured that his blood counts have completely recovered with an absolute white blood cell count of >1500.
The patient was simulated in a supine position with arms down using an immobilization cradle to insure the reproducibility of the position of the neck in relation to the chest as well as the arms’ positions in relation to the chest (Fig. 7.5). The isocenter was placed 2 in. below the sternal notch. Computed tomography (CT) scan was obtained spanning from the mid head to the below the diaphragmatic crus to insure encapture of the entire lung (this is crucial as shown below for the daily verification of the treatment). At our institution and for all patients to be treated for thoracic targets, we do use deep inspiration breathhold (DIBH) technique. Patients are also treated on a 10°–15° incline board to displace mammary tissues in females inferolaterally and away from the radiation field. It also helps displace the heart inferoposteriorly and facilitate DIBH [8]. The patient in case 11 (Fig. 7.5) was coached prior to the simulation for the breathhold by watching a video showing the procedure in details. Respiration was monitored with a video–based, noninvasive system that includes an infrared tracking camera and reflective marker (RPM [real–time position management] system (Fig. 7.6), Varian Medical Systems, Palo Alto, CA). The patient performed DIBH and received feedback via video goggles to improve the stability and reproducibility of breath amplitude.
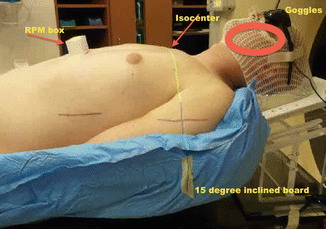


Fig. 7.5
Showing the details of the setup

Fig. 7.6
Showing the RPM box placed on a cartoon illustration
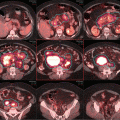
At the time of treatment simulation, 3–4 breathhold–gated non–contrast CT scans were acquired with axial 2.5–mm slices. Imaging data were transmitted to a Pinnacle treatment planning system (Phillips Healthcare) where contouring of the tumor target and surrounding organs at risk as well as treatment planning was performed. Figure 7.7a–c shows the clinical target volume (CTV, red) and planning target volume (PTV, green). Both contours were guided by fusing the CT simulation images with the initial contrast–enhanced CT.
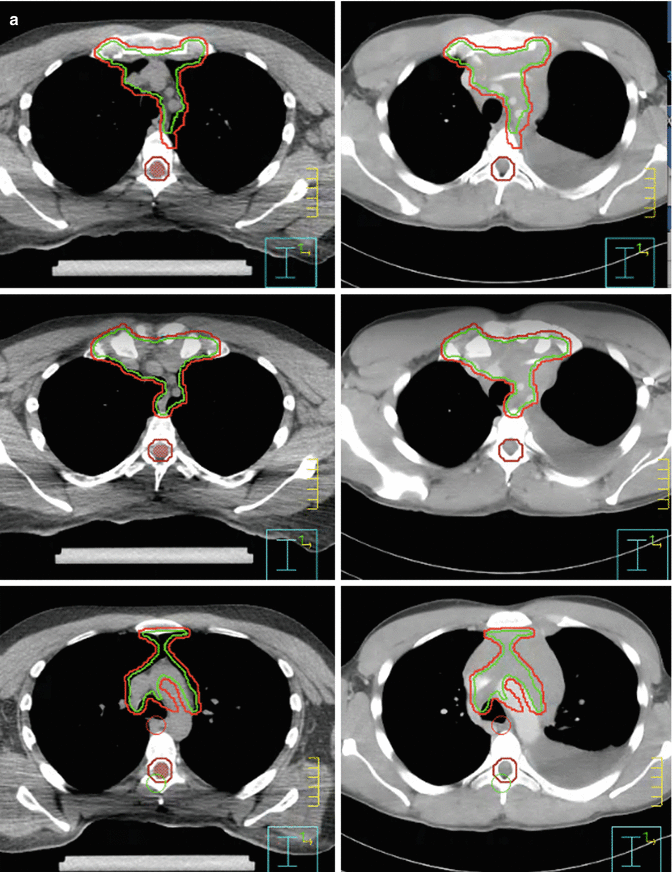
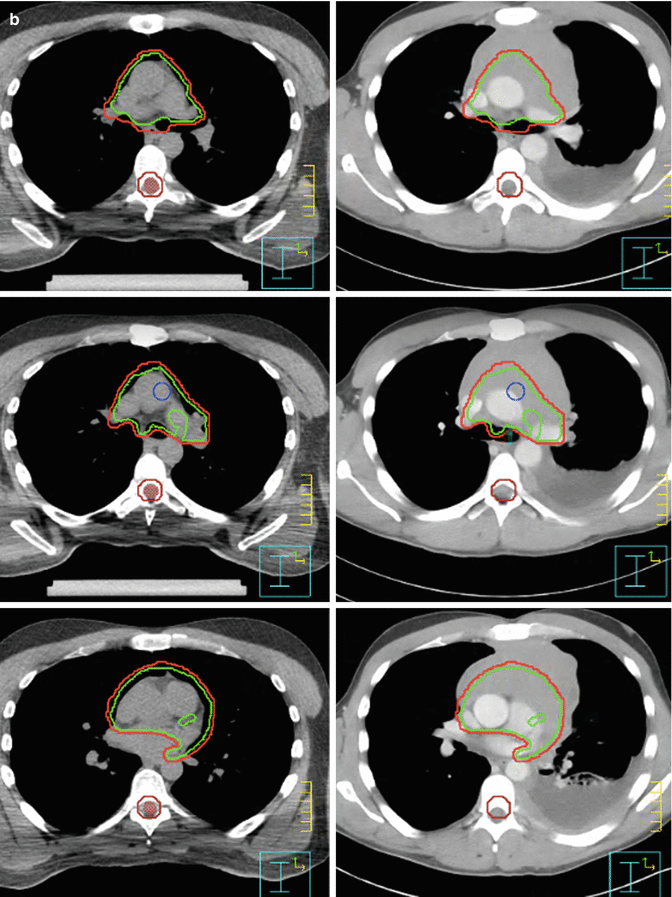
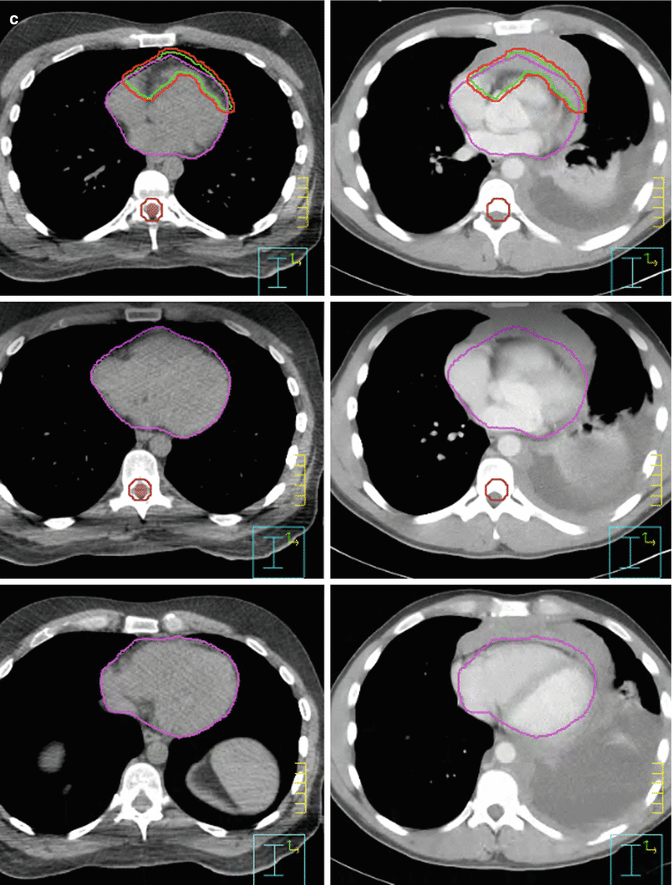



Fig. 7.7
(a, b) Showing the CTV (green) and PTV (red). (c) Showing the CTV (green) and PTV (red). Omitting the part where the mass hanging in front of the heart and avoiding the area of the pleural effusion
IMRT plans were generated with a Pinnacle treatment planning system. Coplanar 6–MV photons were used with 6 beams all anterior–posterior weighted according to our in in–house “butterfly” technique [9] (Fig. 7.8). Involved–site radiation therapy was used for target delineation as per published guidelines from the International Lymphoma Radiation Oncology Group (ILROG) [10].

Fig. 7.8
Showing the beam arrangement used as per the butterfly technique
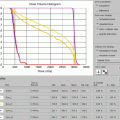
Figure 7.9 shows the IMRT plan generated. Only the mediastinal disease was included in the target volume. There was no attempt to chase the pleural or pericardial effusions. Additionally, the distal part of the mediastinum in the anterior aspect of heart was not included either in an effort to limit heart toxicity. The total dose was 30.6 Gy in 17 fractions. IMRT was delivered by a linear accelerator with step–and–shoot multi–leaf collimation. Low–dose CT on rails was used for daily image guidance (Varian Medical Systems) (Fig. 7.10). Both the lungs and heart structures were delineated on the treatment–planning scan to generate lung and heart dose–volume histogram (generated by the Pinnacle system) (Fig. 7.9c).
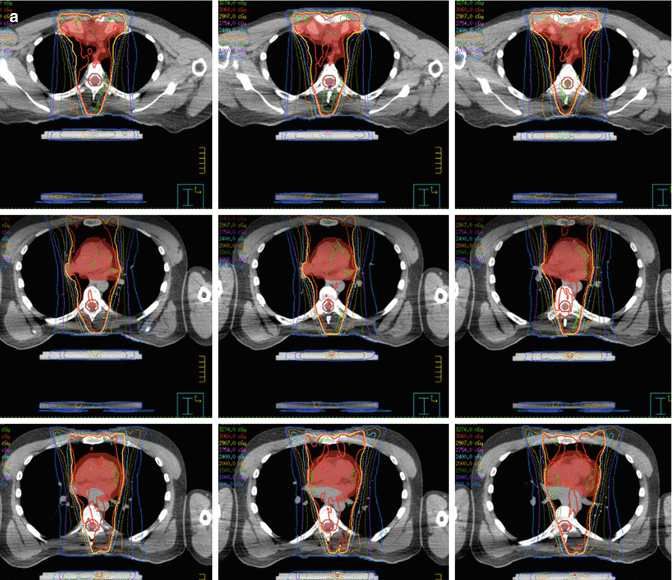
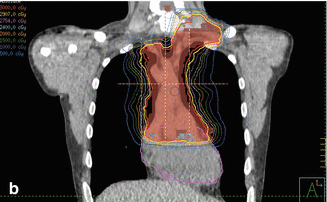
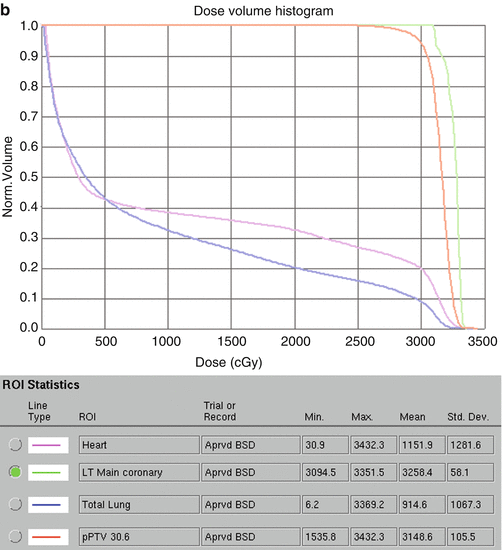
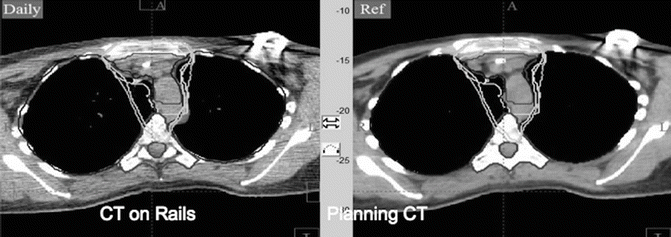



Fig. 7.9
(a) Showing the isodose lines sparing the lung from the low dose by only using anteroposterior beams. (b) Coronal view. (c) Showing the dose-volume histogram for case 1

Fig. 7.10
CT on rail done for verification
Role of Radiation
The clinical presentation with a bulky mediastinal mass makes it appealing to address the local disease with radiation therapy to prevent local relapse. This is an approach that has been adopted in other disease subtypes where mediastinal diseases have a similar presentation, including Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. The evidence for the benefit of radiation in LBL comes mainly from subset analysis in large studies and several retrospective studies. The role of radiation was questioned based on a 47 % mediastinal relapse rate in a publication by Hoelzer et al. [11]. However, among the 45 treated patients, the rate of complete remission was 93 %; out of the 15 (30 %) patients who relapsed, 7 patients relapsed in the mediastinum. Arguably that means that 8 did not relapse in the mediastinum, and also arguably there are 30 patients who did not even relapse and the mediastinal area was controlled. The authors though did not conclude that radiation should not be used. On the contrary they suggested that in certain patients intensification of therapy including chemotherapy and radiation by increasing the dose from 24 to 36 Gy to the mediastinum. One could also argue that may be 24 Gy is not enough to control the mediastinum, as discussed below. Radiation to a dose ≥30 Gy proved to improve both local control and disease-free survival. In spite of that, omitting radiation especially in intensified regimens that included high-dose therapy was tried by GOELAMS. The authors compared re-induction chemotherapy against intensified conditioning followed by stem cell transplantation; they reported 7-year OS and DFS of 64 % and 65 % with little information on the local control [12]. In the recently published GRAALL-LYSA LL03 study, 148 patients (90 % with T-cell LBL) were prospectively treated with a pediatric-like leukemia protocol, with CNS prophylaxis and 2 years of maintenance therapy. There were 34 relapses, yielding a 3-year event-free survival of 63 % and overall survival of 74 %. No mediastinal radiation was given. Of the 131 patients with T-LBL, 30 relapsed and went on to be salvaged. At the last follow-up, 82 of the assessed patients were still in CR. However, there was no information on patterns of relapse and the risk of mediastinal failure.
Our institution has reported different outcomes, and we have highlighted in multiple publications the importance of radiation in local control and disease-free survival.
In a study by Thomas et al., 33 patients with LBL were treated with hyper-CVAD. Seventeen of the 23 patients with mediastinal disease received mediastinal radiation, and only 1 of the 17 relapsed in the mediastinum [13]. In a subsequent publication [14] on 47 patients with T-LBL, none of the 19 patients who received mediastinal radiation had mediastinal relapse, as compared to 8 patients with mediastinal relapse among the 24 patients who did not receive radiation. We updated our experience recently with a more modernly treated cohort. Mediastinal radiation was given to 50 of 77 patients. Mediastinal relapse occurred in 3 of 50 (6 %) patients who received radiation, as opposed to in 7 of 27 (27 %) relapses among those who did not receive radiation. These results translated into an improvement in disease-free survival [15].
The discrepancy in treatment is mainly derived from the fear of toxicity that could be seen with radiation even decades later, especially that these patients who have received intensive chemotherapy. Radiation oncologists should be aware of the treatment-related toxicity as well as of the expectations when treating this disease. The role of radiation is purely to address the mediastinal disease and not to chase after small disease or involvement that might compromise the critical organs, in this case the heart and lung. There have been major technological advances that made it possible for us to target the area of interest while keeping the critical organs at risk safe from radiation [10]. There have been recent data suggesting that lower-dose threshold for critical organs should be used in radiation therapy for hematological malignancies as compared to the ones accepted for solid tumors [16]. Additionally, refining and perfecting planning using modern techniques such as IMRT [9] have made it possible to reduce the volume exposed to the prescription dose. However, robust immobilization and control of internal organ motion such as use of DIBH technique are critical.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree