Fig. 1
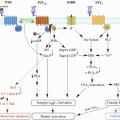
Schematic diagram demonstrating mode of action of new oral anticoagulants (NOAC) on the coagulation pathway
Meanwhile, Factor Xa inhibitors selectively and reversibly bind directly to the active site of Factor Xa (Fig. 1). In the coagulation cascade, Factor Xa binds to Factor Va on the surface of platelets to form the prothrombinase complex which converts prothrombin to thrombin (Eriksson et al. 2009; Cabral 2013). By blocking this interaction, Factor Xa inhibitors prevent the final effects of thrombin generation.
NOACs have predictable pharmacokinetic and pharmacodynamics (Table 1), permitting fixed dosing and eliminating the need for regular monitoring. Although NOACs have minimal food and drug interactions, they are affected by chemotherapeutic drugs that induce or inhibit the P-glycoprotein transport or CYP3A4 pathway (Table 2) (Cabral 2013).
Table 1
Pharmacokinetics and pharmacodynamics of new oral anticoagulants
Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
Target | Factor IIa | Factor Xa | Factor Xa | Factor Xa |
Prodrug | Yes | No | No | No |
Tmax (h) | 1.5–3 | 2–4 | 1–3 | 1–2 |
Half life (h) | 12–17 | 5–9 | 9–14 | 9–11 |
Bioavailability | 6.5 %, pH sensitive | >80 % | >50 % | 45 % |
Protein binding | 35 % | 92–95% | 87 % | 40–59% |
Metabolism | Conjugation | CYP3A4, CYP2J2 | CYP3A4 | CYP3A4 |
Elimination | 80 % renal | 66 % renal (33 % as active metabolites) | 25 % renal | 35 % renal |
Effects of food | Tmax delayed; Cmax & AUC unchanged | Tmax delayed; Cmax & AUC increased | Tmax delayed; Cmax & AUC unchanged | Tmax delayed; Cmax &AUC unchanged |
Drug interactions | Potent P-gp inducers/inhibitors | Potent CYP3A4 inhibitors & P-gp inducers/inhibitors | Potent CYP3A4 inhibitors & P-gp inducers/inhibitors | CYP3A4 inhibitors; P-gp inducers/inhibitors |
Table 2
Interactions between chemotherapeutic agents and immunosuppressants with NOACs based on known metabolic pathway activity
Interaction effect | Dabigatran | Rivaroxaban | Apixaban |
P-glycoprotein | P-glycoprotein | P-glycoprotein | |
CYP3A4 | CYP3A4 | ||
Increases NOAC plasma levels | Cyclosporine | Cyclosporine | Cyclosporine |
Tacrolimus | Tacrolimus | Tacrolimus | |
Tamoxifen | Tamoxifen | Tamoxifen | |
Lapatinib | Lapatinib | Lapatinib | |
Nilotinib | Nilotinib | Nilotinib | |
Sunitinib | Sunitinib | Sunitinib | |
Imatinib | Imatinib | ||
Reduces NOAC plasma levels | Dexamethasone | Dexamethasone | Dexamethasone |
Doxorubicin | Doxoribucin Vinblastine | Doxoribucin Vinblastine | |
Vinblastine |
However, NOACs are not without limitations themselves. In the presence of severe liver and renal dysfunctions, a relatively common feature of patients with cancer, the pharmacodynamics of NOACs may be unpredictable. Dabigatran is excreted by the kidney, and should be used with caution in patients with renal impairment (Cabral 2013). There are no assays to measure the anticoagulation level of NOACs if treatment failure or non-compliance is suspected. The lack of antidote has led to an uncertainty regarding the management protocol in the event of major bleeding or need for urgent reversal of anticoagulation (Prandoni et al. 2014). There is also very little reported about the efficacy or safety of these drugs in children or pregnant women.
6 Treatment of Acute VTE in Patients with Cancers
The aims of acute VTE treatment are to prevent the extension of DVT, prevent fatal PE, recurrent VTE and the subsequent long term complications of VTE such as post thrombotic syndrome (PTS) and chronic thromboembolic pulmonary hypertension. Cancer patients have an annual risk of recurrent VTE of 21–27 % (Hutten et al. 2000; Levitan et al. 1999) despite appropriate anticoagulation with VKA and are 3–4 times more likely to develop VTE than non-cancer patients. The annual risk of bleeding complications whilst on anticoagulation has been estimated to be 12–13 % or 2–6 folds higher than in non-cancer patients (Prandoni et al. 2002).
6.1 Acute Management of Cancer Related VTE
Current guidelines favour LMWH for long term anticoagulation management of acute VTE in cancer patients (Mandala et al. 2011; Farge et al. 2013; Kearon et al. 2012; NCCN 2014; Lyman et al. 2013b). The evidence available to support the routine use of NOACs in the prevention and treatment of VTE remains limited. Six phase III RCTs have been conducted comparing NOACs to conventional anticoagulation therapy on the general VTE patient population (summarised in Table 3) (Schulman et al. 2009; 2014; Bauersachs et al. 2010; 2012; Agnelli et al. 2013a; Hokusai et al. 2013). Cancer patients encompassed 2.5–6 % of trial population. The cancer patients involved in these trials may also not truly reflect the complexity of anticoagulation management in cancer patients, as more complicated patients are more likely to have been excluded. Hence, the results should be interpreted with caution as these trials were not designed and powered specifically for patients with cancer.
Table 3
Trials investigating the safety and efficacy of new oral anticoagulants compared to vitamin K antagonists
Trial acronym | Indication | Study phase | NOAC | Comparator | Total patients | Cancer patients | Treatment duration | Overall death (%) | Recurrent VTE* (%) | CRB (%) |
|---|---|---|---|---|---|---|---|---|---|---|
NOAC vs comparator | NOAC vs comparator | NOAC vs comparator | ||||||||
RE-COVER I 2009 (Schulman et al. 2009) | VTE acute treatment | III | Dabigatran 150 mg bd | VKA | 2539 | 121 (4.8 %) | 6 mo | 1.6 vs 1.7 | 2.7 vs 2.5 | 5.6 vs 8.8 |
RE-COVER II 2013 (Schulman et al. 2009) | VTE acute treatment | III | Dabigatran 150 mg bd | VKA | 2589 | 100 (3.9 %) | 6 mo | 2.0 vs 1.9 | 2.3 vs 2.2 | 5.0 vs 7.9 |
EINSTEIN-DVT 2010 (Bauersachs et al. 2010) | DVT acute treatment | III | Rivaroxaban 15 mg bd for 3wk followed by 20 mg od | VKA | 3449 | 207 (6.0 %) | 12mo | 2.2 vs 2.9 | 2.1 vs 3.0 | 8.1 vs 8.1 |
EINSTEIN-PE 2012 (Buller et al. 2012) | PE acute treatment | III | Rivaroxaban 15 mg bd for 3wk followed by 20 mg od | VKA | 4832 | 223 (4.6 %) | 12mo | 2.4 vs 2.1 | 2.1 vs 1.8 | 10.3 vs 11.4 |
AMPLIFY 2013 (Agnelli et al. 2013a) | VTE acute treatment | III | Apixaban 10 mg bd for 7d followed by 5 mg bd | VKA | 5395 | 169 (3.1 %) | 6 mo | 1.5 vs 1.9 | 2.3 vs 2.7 | 4.3 vs 9.7 |
HOKUSAI 2013 (Hokusai et al. 2013) | VTE acute treatment | III | Edoxaban 60 mg od or 30 mg od | VKA | 8240 | 208 (2.5 %) | 12 mo | 3.2 vs 3.1 | 3.2 vs 3.5 | 8.5 vs 10.3 |
The RECOVER I (Schulman et al. 2009) and RECOVER II (Schulman et al. 2014) were multicentre double-blinded, double-dummy trials involving patients with acute VTE who were randomised to receive either dabigatran or warfarin (target INR 2.0–3.0) following initial parenteral anticoagulation therapy. Pooled data of patients with active cancer in these two trials reported no significant difference in efficacy between dabigatran and warfarin for cancer at baseline (HR 0.75; 95 % CI, 0.20–2.8) or diagnosed during the study (HR 0.63; 95 % CI, 0.20–2.0) (Schulman et al. 2015). The risk of bleeding was not increased by dabigatran. Recurrent VTE occurred more often in patients with (HR 3.3; 95 % CI 2.1–5.3) than without cancer. The authors concluded that dabigatran had a similar efficacy and safety profile to warfarin, with the added benefit or fixed dosage and not requiring monitoring.
The EINSTEIN-DVT (Bauersachs et al. 2010) and EINSTEIN-PE (Buller et al. 2012) were open-label, randomised non-inferiority trials comparing oral rivaroxaban (15 mg twice daily, followed by 20 mg once daily) to standard therapy (subcutaneous enoxaparin followed by vitamin K antagonists) over a period of 3, 6 or 12 months in patients with DVT and PE, respectively.
In a pooled sub-group analysis of cancer patients in both trials, recurrent VTE occurred in 5.1 % of patients in the rivaroxaban arm, compared to 7.1 % of patients in the standard therapy arm (HR 0.69; 95 % CI 0.36–1.33). Corresponding rates of major bleeding occurred in 2.8 % and 5.0 % of patients (HR 0.53; 95 % CI 0.23–1.23). Rivaroxaban was found to have a significantly better net clinical benefit (HR 0.60; 95 % CI 0.36–0.99) in cancer patients.
The AMPLIFY (Agnelli et al. 2013a) and HOKUSAI trials (Hokusai et al. 2013) investigated the efficacy and safety of apixaban and edoxaban, respectively. However, sub-group analysis for patients with cancer was not provided by trial coordinators.
A recent meta-analysis performed subgroup analysis of cancer patients in 6 RCTs comparing the efficacy and safety of NOACs to VKAs for the initial treatment of VTE (Vedovati et al. 2015). The meta-analysis pooled a population of 1132 cancer patients from these 6 RCTs and reported the incidence of recurrent VTE in 3.9 % of patients on NOACs compared to 6.0 % of patients on conventional therapy (Odds Ratio [OR] 0.63; 95 % CI 0.37–1.10). Major bleeding occurred in 3.2 % and 4.2 % of patients receiving NOAC and conventional treatment, respectively (OR 0.77; 95 % CI 0.41–1.44).
Subgroup meta-analysis by Van Es et al. (van Es et al. 2014) reported a significantly lower risk of recurrent VTE with NOAC compared to VKA (relative risk (RR) 0.57; 95 % CI 0.36–0.91; P = 0.02), with no increased risk of major bleeding (RR 0.77, 95 % CI 0.44–1.33; P = 0.35).
Further RCTs that are specifically designed and powered for cancer patients are required. A review of guidelines should be undertaken in light of these findings to consider the cost-effectiveness of NOAC in the management of acute VTE in patients with cancer.
6.2 Extended Treatment of Cancer Related VTE
There is no consensus on the duration of anticoagulation following the diagnosis of VTE. The American College of Chest Physicians (ACCP) and National Comprehensive Cancer Network (NCCN) recommend that treatment of VTE should be continued for 3 months with annual reassessment of risk of thrombosis (Kearon et al. 2012; NCCN 2014). However, American Society of Clinical Oncology (ASCO) recommend at least 6 months of anticoagulation in patients with metastatic disease, receiving chemothreapy or recurrent thrombosis (Lyman et al. 2013a). Although LMWH has been found to significantly reduce the risk of VTE compared to VKA (Akl et al. 2011a), this is not a preferred method for patients due to daily injections. Four RCTs have been conducted comparing the safety and efficacy of extended treatment of VTE with NOACs to conventional therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree