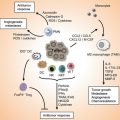
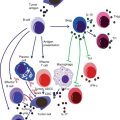
Fig. 8.1
Fine modulation of cellular recruitment by chemokines. The chemokine network is organized around several levels of complexity. (a) Most of the cell types (1, 2, 3) express several chemokine receptors and a same receptor is found on several cell types. Moreover, different chemokines can bind to a same receptor and most of the receptors can bind several chemokines with distinct affinity (color gradient represent differential affinity). This apparent complexity allows for the fine control of cell population recruitment. (b) The schematic representation illustrates the selective recruitment of cell populations according to the respective colored CK gradient. The number of cell recruited is related to the affinity of the respective CK for its receptor
Cancer constitutes a very complex pathology in many aspects. Neoplastic cells result from the environmental, viral-induced, or inherited deregulation of genes known as “oncogenes” or “tumor suppressor genes.” This primary modification often leads to uncontrolled expansion of undifferentiated cells for which the transcriptome and the proteome are highly modified in comparison with the original cell. Nevertheless, it is important to note that tumor development does not result from the simple expansion of neoplastic cell. Indeed, solid tumors (primary tumor as well as metastasis) are also constituted by a wide variety of stromal cells. Stroma is composed of nonhematopoietic cells, such as “healthy” cells of the affected tissue, fibroblasts, or endothelial cells, as well as hematopoietic cells. Hematopoietic cell populations are mainly composed of innate immune cells, such as tumor-associated macrophages (TAMs), dendritic cells (DCs), natural killers (NK cells), neutrophils, and partners of the adaptive immune response such as T and B lymphocytes.
The relative importance of the stroma compared to tumor cells depends on the type of cancer [9], but it is now well described that several stromal cells are important predictive markers of cancer evolution (macrophages, regulatory T cells, and endothelial progenitor cells). Even though the stroma cannot be characterized properly in circulating hematological tumors, leukocytes will have an important impact on the expansion, survival, and potential homing of tumor cells to the specific tissue. This phenomenon is distinguishable from the metastatic process where the tumor cells need to cross the endothelial barrier from a primary tumor site and home to a distant tissue. The stroma contributes to the global organization and progression of the tumor known as “tumor microenvironment” through the production of growth factors, cytokines, CKs, exchange of nutrients, and tissue remodeling and repair. In contrast, immune cells are responsible for the control of tumor growth. The concept of immunosurveillance proposed by Burnett et al. [10] in the early 1970s has been widely debated. Recently, Schreiber and colleagues provided experimental evidence for the clinical emergence of cancer as a result of strong selection and modeling of tumors by the immune system in a process termed as “tumor editing” [11]. In this process, neoplastic transformation occurs, and tumor cell expansion is detected by the innate and adaptive immune systems, which either succeed in complete tumor elimination or maintain a state of equilibrium between tumor cell expansion and elimination. This phase leads to the immune selection of tumor cell variants that develop immune resistance and immunesuppressive mechanisms resulting in tumor escape and cancer progression to a clinical outcome.
Cancer is a complex process whereby undifferentiated tumor cells expand locally in specialized tissues, migrate in an active manner by leaving the primary tumor site through the endothelial barrier, establish in a distant and different specialized tissue and finally generate metastases. Inflammation generated by neoplastic transformation contributes to the recruitment of protumoral population and the production of growth factors as well as the recruitment of immune component with antitumor activity. Thus, tumorigenesis is a dynamic process involving important tissue remodeling and angiogenesis, recruitment and local migratory mechanisms, and survival and cell death for both tumor and stromal cells in which the CK/CKR network has major implication.
The CK/CKR network appears to be a promising target in cancer therapy and has already been used in standard therapeutic approaches, as well as in immunotherapy. Numerous basic and clinical interventions rely on the development of agonist or antagonist CKR in order to manipulate their critical biological function toward antitumor activity.
In this chapter, the role of the CK/CKR network in these aspects of cancer development, as well as its potential application in the improvement of cancer therapy, is described in detail.
8.2 Chemokines and Chemokine Receptors
Chemokines are small cytokines initially described for their chemotactic properties on leukocytes. During cell recruitment from the blood to inflamed tissues, CKs initiate the activation of circulating cells, promoting cell rolling, adhesion to activated endothelium, and extravasation (Fig. 8.2). In tissues, CKs determine cell directional migration, by establishing a concentration gradient (Fig. 8.3). Evidence from previous studies has shown that the control of cell mobility by CKs is implicated in developmental mechanisms and cell homeostasis, as well as in the induction and tuning of acute and chronic inflammation and control of the immune response. Numerous reviews have extensively described the CK classification, structural organization, and their associated biological properties [12, 13]. CKs are subdivided in four subfamilies based on the number and spacing between conserved cysteine in the primary amino acids sequence [14]. CKRs are seven transmembrane G-protein-coupled receptor classified according to the CK family they bind. As previously mentioned, most CKs bind to several receptors, and most of the receptors can bind several CKs with different affinities. Additionally, one cell subset can express different CKR and the same CKR is expressed by different cell subsets. This apparent redundancy is in reality a tool to tightly regulated leukocytes, stem cells, and other cell types’ migrations during physiological and pathological condition.
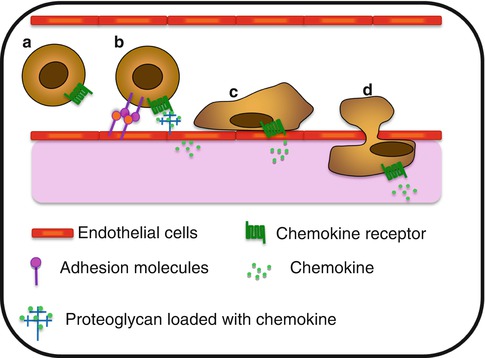
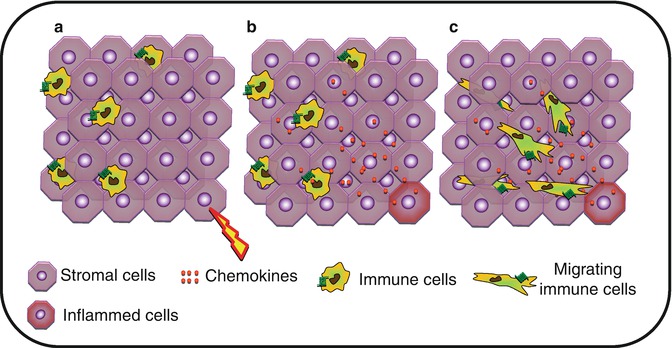

Fig. 8.2
Chemokine-associated extravasation process. (a) Circulating cell within the bloodstream. (b) Chemokine presented by proteoglycan on activated endothelial cells, induce the expression of adhesion molecules implicated in the slow rolling and the capture process. (c) Once stuck to the endothelium, cell exerts crawling behavior on the luminal side of the blood vessel and (d) extravasates and migrates through the tissue toward a chemokine gradient

Fig. 8.3
Interstitial migration. (a) Upon activation, (b) stromal cells will produce chemokines forming a gradient within the tissue. (c) Tissue-infiltrated immune cells will migrate through the tissue toward the higher concentration of chemokine
It is now well established that CK function is not limited to cell migration. It has been clearly demonstrated that CKs directly control cell proliferation, survival and senescence, as well as cytokine secretion and phagocytic properties (Fig. 8.4). It is the balance between these migratory, secretion, phagocytic, survival, and proliferation signals which explains the central roles of CK in development, tissue homeostasis, repair, inflammation, and immunity.
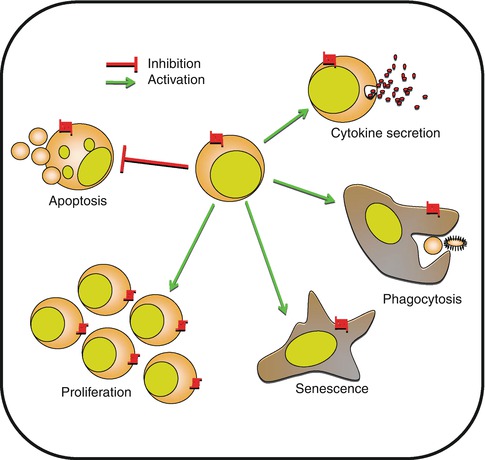

Fig. 8.4
Control of cell biology by chemokines. Besides cell migration, chemokines are implicated in multiple cellular functions including apoptosis, proliferation, and senescence. Chemokines are also directly implicated in cell activation, cytokine secretion, or phagocytosis
8.3 Control of Tumor Cell Behavior
The biological property controlled by the CK/CKR recognition system is not restricted to chemotactism. Several important processes involved in the behavior of tumor cells will be affected by these axes. In this section, the effect of CK/CKR expression on tumor cell behavior and cancer progression is discussed.
8.3.1 Chemokines and Chemokine Receptor Alterations During Neoplastic Transformation
Primary neoplastic transformation leads to strong modification of the transcriptome and proteome which is mainly shaped by immune selection of resistant tumor variants. CK and CKR are not oncogenes per se; however, modulation in the production of CK or their receptors by tumor cells is often the result of oncogenic modifications and immune selection (Fig. 8.5). The first evidence came from a human papillary thyroid cancer. The authors showed that RET (rearranged during transfection)-tyrosine kinase rearrangement promotes the secretion of numerous inflammatory cytokines, including CCL2, CCL20, and CXCL12, and increases the expression of CXCR4 [15]. Later studies have shown that Myc overexpression in pancreatic cancer has been associated with increased CK expression [16, 17]. Nevertheless, the predictive outcome of oncogenic modifications on the regulation of CK and CKR expression is difficult to assess. While RAS-RAF signaling pathway promotes CXCL8 and CXCL1 transcription in pancreatic and ovarian cancer, it inhibits CCL27 transcription in skin cancer [18–20]. Similarly, Von Hippel-Lindau tumor suppressor mutation in renal cancer [21] and TP53 mutation in cancer stem cells promote CXCR4 expression [22] while downregulating its expression in breast cancer cells [23].
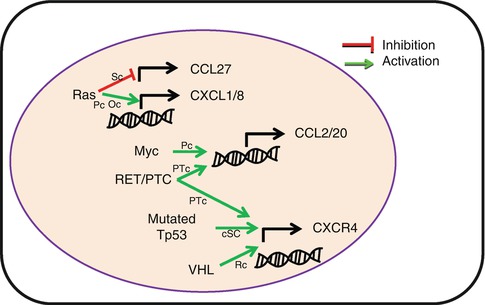

Fig. 8.5
Oncogenes induce altered chemokine and chemokine receptor expression by tumor cells. Common oncogene mutations are associated with modification of chemokine or chemokine receptor transcription, resulting in tumor promotion. RET/PTC rearranged RET tyrosine kinase, VHL Von Hippel-Lindau tumor suppressor gene, Sc skin cancer, Pc pancreatic cancer, Oc ovarian cancer, HPTc human papillary thyroid cancer, cSC cancer stem cell, Rc renal cancer
Through modification in the profile of CKR expression, tumor cells will change their sensitivity to the microenvironment and acquire new migratory and homing capabilities.
8.3.2 Metastasis/Homing
The metastasis index is undoubtedly the major factor of prognosis and determines the therapeutic attitude. Metastasis defines the process through which tumor cells leave a primary site to settle in a distant location and creates a new colony. This phenomenon is a characteristic of tumor malignancy including tumor invasion, intravasation, and homing to different sites. This has to be distinguished from the potential secondary localization of circulating tumor cells which only involves the homing mechanism.
8.3.2.1 Tumor Invasion
The first step of metastasis spreading relies on either tumor cell or stromal cell-mediated fibrosis activity and the ability of tumor cells to acquire migration and intravasation capabilities, in order to leave the primary tumor site and reach the bloodstream. Chemotaxis of tumor cells is well characterized [24]. This process requires a paracrine loop between tumor cells and stromal cells, such as macrophages shaping the microenvironment to favor metastasis [25]. Different chemical gradients may induce tumor cell chemotaxis, but the direct implication of CKs in this specific process is poorly documented. We can distinguish the indirect contribution of CK to the chemotaxis activity of cancer cells through angiogenesis, fibrogenesis, and matrix remodeling mediated by stromal cells.
CXCL12/CXCR4 is the major axis directly involved in tumor cell metastases. Overexpression of CXCR4 in rat mammary adenocarcinoma enhances the motility of tumor cells in the primary tumor [26]. This receptor is widely involved in the epithelial-to-mesenchymal transition (EMT) process, which is a major step leading to metastasis [27, 28]. Few studies have reported the implication of other CKs and CKRs such as CCL18, CCL2, or CXCR7 [29–31] through the activation of EMT-implicated signaling pathways. IL8/IL8R axis might also favor maintenance of the mesenchymal status of the tumor cell [32]. Interestingly, the integration of multiple CKR axes adds complexity to the tumor invasion process. Indeed, overexpression of CXCR4 promotes invasion. However, coexpression of CXCR7 which binds the same ligand CXCL12 impairs invasion but favors angiogenesis and primary tumor growth [26].
8.3.2.2 Homing
Once in the bloodstream, the tumor cell needs to migrate to a site that will allow its engraftment, survival, and proliferation. In 2001, Muller et al. demonstrated for the first time that the expression of specific CKRs by tumor cells could predict the implantation of malignant cells in tissues expressing high levels of the receptor ligands [33]. Since then, several other studies have established associations between metastases, CKR expression, and implantation sites for various cancer types (Table 8.1). Consistently with their homeostatic functions, CCR7 expression by tumor cells is associated with lymph node metastases; CCR10 with skin metastasis; CX3CR1 with brain, liver, and bone metastases; CCR9 with intestine metastases; and CXCR4 with bone and liver metastases [33–36].
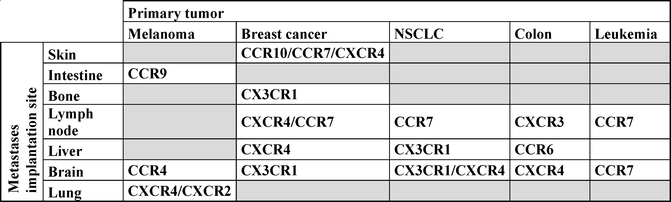
Table 8.1
Metastases implantation of various cancer types based on their chemokine receptor expression

Overall, these observations show that CK axes generate a complex relationship between tumor cell and the environment and deserve further attention in preclinical studies as it represents an important target with clinical application.
8.3.3 Senescence, Proliferation, and Survival
Tumor expansion results in the capacity of tumor cells to proliferate infinitely without developing senescent mechanisms. Several CKs have demonstrated the ability to activate signaling pathways in favor of this goal.
Cellular senescence is generally defined as an irreversible state of G1 cell cycle arrest in which the cell is refractory to growth factor stimulation. Activation of CXCR2 by either CXCL1 or CXCL8 can result in senescence induction [37]. CXCR2 activation is thus able to act as a suppressor of malignancy in prostate and breast cancer [38, 39].
Inhibition of tumor proliferation by CXCR2 ligand is probably limited to tumor models and to early stages of tumor development. Indeed, the same CK axes display opposite effects in other tumor models. CXCR1 and CXCR2 activation by CXCL8 promotes the proliferation of gastric cancer, esophageal cancer, non-small lung cancer, and melanoma cell lines [40–43]. Other receptors of the CXC receptor family are involved in tumor cell proliferation. CXCR6 is involved in cell proliferation of pancreatic cancer cells [44], and CXCR4 is associated with tumor proliferation in numerous models, including ovarian, melanoma, glioma, renal, lung, and thyroid cancer cells [27, 45]. Few studies have investigated the implication of CCRs in the control of tumor cell proliferation. CCR6 favors colon tumor cell proliferation upon CCL20 activation [46], and CCR9 favors pancreatic cancer cell proliferations upon CCL25 activation [47].
Another role of CK in tumor cell biology is the ability to control tumor cell survival, essentially mediated through the CC receptor family. CCR10 activation promotes phosphatidylinositol-3-kinase-mediated protection from apoptosis of melanoma cells [48]. The same mechanisms are observed in squamous cell carcinoma of the head and neck after CCR7 activation [49]. CCR7 engagement by CCL21 is also implicated in the prevention of apoptosis in NLCLC, through ERK-dependant activation pathways [50].
CK direct promotion of tumor cell survival is not limited to CC chemokines; CXCL12 through CXCR4 activation promotes hepatoma, ovarian, and chronic leukemia tumor cells survival [51], and CXCR7 activation increases cell survival by reducing apoptosis [52].
Overall, these observations highlight extended functional contributions of the CK system to tumor development and reveal that they are not merely restrained to chemotaxis.
8.4 Control of Immune Cell Behaviors
As described previously, the immune system is known to shape the tumor through the “tumor editing” phenomenon. In this context, CKs are directly or indirectly implicated in the control of immune cell activation, migration to the priming site, and immune response induction. It is now clear that in most cases, the CK network is shunted by the tumor, favoring its escape from immunosurveillance and tumor progression. Nevertheless, the production of some CKs promotes the antitumor immune response and has been associated with improved patient outcome, including lower recurrence rate or increased patient survival [53].
8.4.1 Chemokines Involved in T-Cell Antitumor Immune Response
Induction of antigen (Ag)-specific antitumor immune response requires the uptake of tumor Ag by professional antigen-presenting cells (APCs) and migration from the tumor site to the corresponding draining lymph node, in order to present the processed tumor-Ag to T lymphocytes. These major immune functions can be divided into different steps for which the CKR network has important regulatory implications [54].
8.4.1.1 Migration of APCs to the Priming Site
Encounter with tumor Ag induces maturation of APCs present in the tumor environment. One feature of this maturation is the downregulation of peripheral tissue-associated CKR like CCR1, CCR5, and CCR6 and the upregulation of CCR7. Due to the constitutive expression of CCR7 ligand, CCL19, and CCL21 by peripheral lymph nodes, this switch of CKR expression by APCs promotes their migration toward the priming site. Once in the draining lymph node, APCs will locate in the preferential area to present the tumor Ag to the CCR7 expressing naive lymphocyte.
8.4.1.2 Ag Presentation to T Lymphocytes
Despite the fact that APCs display low dynamic activity, naïve lymphocytes have a high basal mobility favoring scanning of thousand APCs per hour [55, 56]. This behavior requires CCR7 expression by T lymphocytes [57]. An additional CKR-dependant mechanism favors the probability of encounter between APCs and T lymphocytes. Encounter of Ag-specific CD4+ or CD8+ T cells with an APC bearing their cognate Ag induces the secretion of CC-chemokines by the conjugate, namely, CCL19, CCL5, CCL3, and CCL4. These CKs will promote naïve T-cell scanning behaviors and attraction toward the conjugate [58–60], which is known to favor the establishment of memory immune response, in addition to the induction of polyclonal responses against different tumor Ags [61].
CKs are also implicated in the improvement of APC/T-cell adhesion mechanism as well as in immunological synapse stabilization, promoting T-cell priming (Fig. 8.6). CCR7 ligands secreted in the lymph node promote immunological synapse formation by T cells [62]. CXCR4 and CCR5 expressed by T cells are recruited toward the immunological synapses made with the APC. This polarization results in desensitization of T cells from external sources of CKs and improves synapse stability. A similar mechanism is observed during the interaction between tumor-infiltrated lymphocytes (TILs) and tumor cells. Indeed, the recruitment of CCR5 at the immune synapse formed between the TIL and the tumor cell results in defective responses to TIL toward a CCR5 gradient [63]. This mechanism allows for the modulation of the “GO” signals generated by CKs, competing with the “STOP” signals mediated by the TCR-MHC interaction [64].
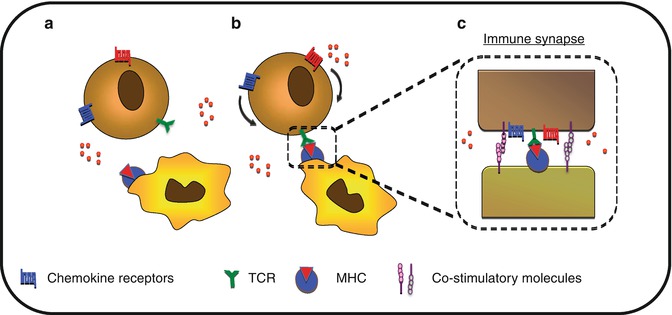

Fig. 8.6
Control of cell polarization toward immune synapse. (a) T-cell scan for their cognate antigen-presenting cell. (b) Upon recognition, T cell will polarize chemokine receptors toward the immune synapse. (c) This sequestration of CKR leads to reduced sensitivity to distant CK gradient and may participate in the stabilization of the immune synapse
8.4.1.3 Migration of Effector T Lymphocytes to Tumor
Naive T cells, after clonal expansion and differentiation into effector T cells, migrate toward the tumor site, implying that T cells downregulate the expression of the CKRs implicated in the retention at the priming site like CCR7. In addition, they upregulate various CKRs including CCR1, CCR3, CCR5, and CXCR3 allowing their movement toward the tumor site [65]. Cytotoxic T lymphocytes (CTLs) recruitment to the tumor site is consistent with this pattern of CKRs expression and is mainly mediated by CCL3, CCL5, CCL20, CXCL9, and CXCL10 [54]. Membrane-anchored CKs expression such as CXCL16 and CX3CL1 have also been shown to correlate with greater numbers of tumor-infiltrated lymphocytes and improved prognosis in colorectal cancer [66, 67]. The antitumor effect of the membrane-bound CK form vs. the soluble form is yet to be clearly established.
The control of TIL localization within the tumor is ill-defined. It is obvious that in most cases, TILs are mainly found at the tumor periphery; however, the underlying mechanisms remain unclear. Several clues could help us speculate on the mechanism of trapping the TILs at the tumor periphery. The recent contribution of real-time imaging showed that dense peripheral extracellular matrix might restrain TILs’ access to the tumor parenchyma [68]. Whether specific niches of CKs are expressed on collagen fibers is unclear and needs further investigation. In addition, dynamic analysis showed that Ag-specific CTLs are trapped in the network of tumor-associated APCs restraining their infiltration and probably favoring immunosuppression [69, 70]. The role of CKs in this trapping is not defined, but Ag expression by APC at least induces stable engagement between the CTL and the APC. In addition, experimental evidences showed that non-tumor-Ag-specific TIL cannot infiltrate the tumor deeply without the prior tumor cells’ destruction by Ag-specific CTL. These results suggest that deep infiltration of the tumor by TIL might be favored by chemotactic agents secreted upon tumor cell destruction by CTL or on extensive ECM remodeling to allow their interstitial migration [71].
Overall, considering the numerous CKs expressed by the various cell subsets of the tumor microenvironment, it is very difficult to address specific contributions of CK/CKR couple in the interstitial migration and positioning of T lymphocytes within the tumor parenchyma. The various properties of these molecules have demonstrated that this positioning is controlled by sensitivity to the chemotactic gradient and the subsequent desensitization upon polarization toward the synapse or the downregulation of the expression of CKRs.
8.4.2 Chemokines in Innate Immune Components
Innate immune cells constitute a first barrier against tumor development. However, due to their plasticity and capacity to produce a myriad of cytokines, chronically activated innate immune cells are key modulators of cell activation and survival, as well as regulators of the ECM metabolism. Several physiological processes necessary for tumor development, such as increased cell survival, tissue remodeling, angiogenesis, and suppression of antitumor adaptive immune responses, are regulated by innate immune cell infiltrate in the tumor.
Macrophages are the main stromal cell population present in the tumor parenchyma. They can account for more than 50 % of the tumor mass. The role of TAM in tumor development is critical, as these cells, depending on their state of activation, can display antitumor properties associated with production of Th1 cytokine, high quantity of reactive oxygen species, and efficient Ag presentation or they could display protumor properties mediated by the secretion of Th2 cytokine, proangiogenic factors, growth factors that support tumor survival, and proliferation and the secretion of MMP which promote tumor invasion and metastases. Consistently, the impact of TAM on tumor development and metastases will depend on the balance between M1 antitumor macrophages and M2 protumor macrophages.
Depending on the tissue, resident macrophages are in a small proportion derived from the recruitment of circulating monocytes assuring immunosurveillance and mainly origin from self-renewal of interstitial resident macrophages derived from the yolk sac or fetal liver [72]. Within neoplastic tissues, it is suggested that TAMs are mostly recruited from the periphery. Nonetheless, knowledge of the relative proportion of native resident macrophages remains a poorly investigated field in oncology. CCL2, also called MCP-1 for monocyte chemoattractant protein-1, is probably the most frequently found CC-CK in tumors involving recruitment of circulating monocytes [73]. Interestingly, in a melanoma system where tumorigenesis is dependent on an external growth factor CCL2, there is a biphasic effect depending on its secreted quantity. High amounts are associated with a massive recruitment of TAM into the tumor with dominant antitumor activity, while lower amounts induce lower infiltration into the tumor resulting in tumor promotion through the secretion of growth factor by the macrophages [74]. These results point out the importance of the ratio between protumor and antitumor macrophages recruited into the tumor.
Other CKRs implicated in TAM recruitment are CX3CR1 and CCR1. In human glioblastoma, the level of tumor infiltration by microglial cells is dependent on CX3CR1. Patients with a functional mutation in the CX3CR1 gene associated with impaired monocyte migration have a reduced TAM infiltration into the tumor [75]. Injection of a thymoma tumor cell line (EL4) with a liver tropism to mice results in an increased infiltration of the liver by immune cells, including macrophages. In CCR1 KO mice, this recruitment during the first stage of the tumor development is massively reduced [76].
CXC chemokine receptors could also be implicated in TAM recruitment. In humans, IL-4 and IL-13, two cytokines secreted in the tumor environment, sensitize monocytes to CXCL1 and CXCL8 by upregulating their receptors (CXCR1 and CXCR2). Thus, these cytokines indirectly promote the recruitment of TAM into the tumor through CXC chemokine receptors [77].
As previously discussed, CKs not only control leukocyte recruitment into the tumor but also organize their localization within the tumor. Lack of proper vascularization at the center of the tumor induces the secretion of several hypoxic factors like hypoxia-inducible factors (HIFs). HIFs promote the expression of CXCR4 by macrophages, favoring their recruitment toward tumor hypoxic areas [78]. On the other hand, tumor environment decreases CKR expression on monocytes. Indeed, macrophages from tumor sites express low levels of CKR [79]. Time-lapse imaging of TAMs in experimental murine model revealed that TAMs display reduced displacement but intense protrusive activity [69, 70]. Downregulation of CKR might explain this retention at the tumor site.
CKs do not only act on leukocyte attraction but are also implicated in their activation. Induction of copper/zinc-superoxide dismutase by CCL5/CCR5 activation causes tumor necrosis factor-alpha and reactive oxygen species production by macrophages [80], promoting tumor destruction. Inversely, in human monocytes, CC chemokines induce the transcription of metalloproteinase, implicated in tumor invasion and spreading. The fact that both TAM recruitment and activation are regulated by CK increases the potential interest of targeting TAM for antitumor therapies.
NK cells represent another component of the innate immune system highly involved in antitumor immune responses. NK cell recruitment to the tumor is mainly mediated through the CXCL10-CXCR3, CX3CL1/CX3CR1, and CCL3-4-5/CCR5 axes. High CX3CL1 quantity is associated with increased NK cell recruitment into the tumor in both human and mice [81, 82]. Similar phenomenon is observed with increased CCL5 and CCL3 expression by tumor cells in mouse models [83, 84]. CXCR3 is implicated in the recruitment of human NK cells to breast cancer tumor, which is mediated by CXCL10 secretion from tumor cells in response to IFN-γ produced by the NK cells themselves [85, 86]. Thus, CKs not only control NK cell recruitment but also regulate their antitumor properties. CX3CR1 activation by CX3CL1 results in improved antitumor cytotoxicity of NK cells [87, 88]. CCL3, CCL4 and CCL5 have been shown to activate NK cytotoxicity through induction of degranulation [89, 90].
8.4.3 Chemokine and Tumor-Induced Tolerance
Recruitment of tolerogenic cells such as regulatory T cells or immunosuppressive myeloid subsets is a feature or immune escape. Tumor cells secrete ligands of CKRs expressed by immature, regulatory or Th2 polarized cells. CCL22 and CCL17 produced by tumor cells recruit monocytes, as well as Th2 lymphocytes and regulatory T cells through CCR4 signaling [91]. This strategy of immune escape has been also selected in viral-induced oncogenesis process. HHV8 virus, the pathogen of Kaposi’s sarcoma, encodes three viral CKs which bind to CCR3, CCR4, and CCR8 involved in the recruitment of Th2 and regulatory T cells [92].
Stromal cells produce CKs which promote the recruitment of protumoral cells. Amongst others, TAM produces CCL18 which is induced by IL10 [93]. CCL18 favors the recruitment of naïve T cells through activation of an unknown receptor. It is proposed that these naïve T cells acquired tolerogenic properties in contact with the tumor environment. CCR6+ immature lymphoid DCs recruitment into the tumor is favored by the secretion of CCL20 from both tumor cells and TAM [94]. CCL5 recruits immature DCs as well by binding CCR1 and CCR5 [95]. Immature DCs acquire tolerogenic properties in the tumor environment and participate in the immune tolerance loops against tumor Ags [96].
Subversion of tumor immune component is a central point of tumor outcome. The above described implication of CK in cellular mechanisms should provide the basis to better understanding the clinical implication of CK network in cancer pathology. The regulation of the balance between immunogenic and tolerogenic components has deserved major attention for a long time and is the basis of immunotherapy which represents an apparent inexhaustible field of innovative anticancer strategies. Targeting the CK system in this goal is in the course of important investigation through the development of pharmaceutical compounds able to stimulate or antagonize CKR axes.
8.5 Alternative Tumor-Associated Physiological Functions of Chemokines
8.5.1 Angiogenesis
One of the features of CKs is their dual role in the angiogenic process. In the tumor environment, there is increased production of proangiogenic CK, while angiostatic CKs are downregulated. In addition to a direct angiogenic effect of CKs, this activity is indirectly potentialized by the CK-induced recruitment of leukocyte displaying angiogenic properties such as neutrophils or macrophages [97].
CK from the CXC family are probably the most described for their direct implication in tumor-associated angiogenesis. CXCLs 1, 2, 3, 5, 6, 7, and 8 display angiogenic properties. All these CKs contain a specific amino acid sequence of glutamic acid-leucine-arginine (or ELR for short) immediately before the first cysteine of the CXC motif (ELR-positive). This ELR sequence absence from the other CXC chemokines is responsible of the proangiogenic properties of most of the CXC chemokine [98].
ELR+ chemokines mediate angiogenesis through binding to the CXCR2 receptor. ELR+ chemokines are able to recruit endothelial precursor cells, induce cell proliferation, and promote maturation. These mechanisms could be negatively regulated by a decoy CKR expressed by endothelial cells called duffy antigen receptor for CK (DARC). Unlike most of the other CKR, DARC is not linked to G protein, and its activation does not induce calcium flux. DARC reduces angiogenesis by sequestering all the ELR+ CKs.
One specificity within ELR− chemokines is attributed to CXCL12 which is the only ELR− chemokine with proangiogenic activity. CXCL12 mediates its proangiogenic effect by directly promoting the recruitment of endothelial progenitor cells [99, 100] or indirectly by promoting tumor angiogenesis through the recruitment of CXCR4+ proangiogenic monocyte [78, 101] and through the secretion of vascular endothelial growth factor (VEGF) by CXCR7 activation [102].
In contrast, ELR− chemokine secretion is often associated with attenuation of angiogenesis. ELR− CXC chemokines are described by their angiostatic properties. ELR− CXC chemokine secretion is induced by IFN-α and IFN-β. Through CXCR3 binding, these CKs mediate their angiostatic properties by inhibition of ELR+ chemokine, VEGFα, and βFGF proangiogenic effects in vitro [103]. Interestingly, the expression of CXCR3 is dependant of the cell cycle phase, limiting the angiostatic properties of ELR− CXC chemokines to the S/G2-M phase [104].
This important association of CKs and angiogenesis within the tumor environment sets the inhibition of ELR+ chemokine as a robust antitumor therapy.
8.5.2 Fibrosis and Extracellular Matrix Remodeling
The association of CKs in EMT leading to fibrosis activity has been previously suggested by studies; however, there is no clear evidence that CKs play a direct role in this process.
Fibrosis and extracellular matrix remodeling are continuous processes present in the tumor parenchyma reflecting the intense dynamic and migratory activity of the neoplastic tissue. Two different types of migratory activity are defined, namely, the amoeboid and mesenchymal migration. The amoeboid migration does not require extracellular matrix (ECM) remodeling through matrix metalloproteinases (MMPs) activity due to the ability of the cell to squeeze through the ECM. The mesenchymal migration relies on previous proteolysis and degradation of the ECM to generate sufficient space for cell displacement. CK-mediated induction of MMP is mostly mediated by CC chemokines; CCL5 and CCL9 produced by mesenchymal stem cell promote tumor cell invasion in a MMP-dependant manner [105, 106]. CCL25 promotes MMP secretion in ovarian cancer cells through CCR9 binding and favors tumor cell invasion [107]. CCL21/CCR7 interaction favors MMP-9 secretion, tumor invasion, and metastases in colon cancer cells and in B-cell chronic lymphocytic leukemia cells [108, 109]. At least, one CXC chemokine has been related to MMP activity; thus, CXCL12 is implicated in increased MMP2 activation and increased cell invasion in a pancreatic cancer cell line [110].
Studies have suggested that the extracellular matrix promotes tumor escape from the immune system by trapping antitumor leukocytes at distance from tumor cell niches [111]. However, tumor progression and metastases require degradation of this extracellular matrix surrounding the tumor. The main protagonists of these physiological activities are represented by mesenchymal stem cell (MSC)-derived cell populations. CXCL12 is implicated in the recruitment of mesenchymal stem cells (MSCs) from the bone marrow. Bone marrow-derived MSCs can account for up to 25 % of the cancer-associated fibroblasts, the main source of fibrosis within the tumor [112].
There is ongoing evidence that targeting proteolysis activity in combination with chemotaxis would provide promising results in the strategy to inhibit tumor cell invasion and metastasis.
8.6 Clinical Aspect
CKs are implicated in several aspects of tumor development. Due to these pivotal roles in tumor biology, CKs have been frequently associated with tumor evolution and clinical outcomes and have been highlighted for their potential use as prognostic or diagnostic markers. Therefore, they represent a promising target with a potential for a diverse range of therapeutic strategies.
8.6.1 Prognosis
Due to its importance across a wide range of physiological mechanisms, CK/CKR network alteration could impact tumor development. Correlative studies using genetic polymorphisms provide essential information for prognosis. Several functional polymorphisms in CKs or CKRs have been studied in order to establish correlation between functional variants and tumor risk or progression (Table 8.2).
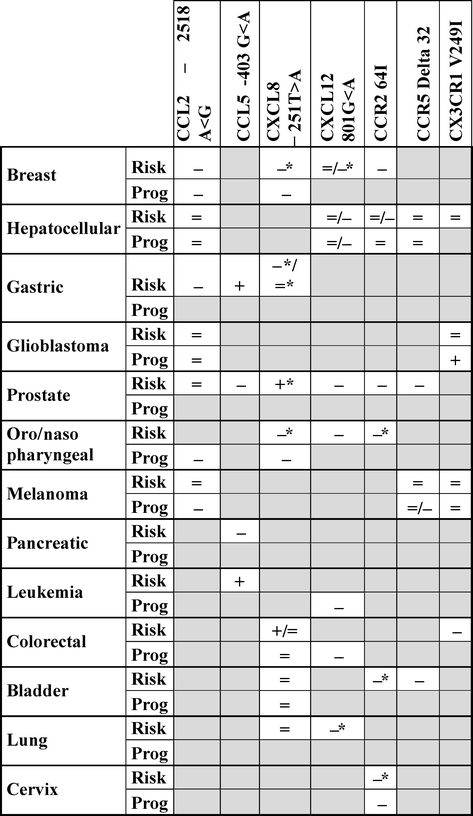
Table 8.2
Association between chemokines and chemokine receptor polymorphisms and tumor risk and/or progression

The paragraphs below focus on the most commonly described polymorphisms, their functional relevancies, and their subsequent prognostic value in tumor risk and/or progression.
8.6.2 CC Chemokines/Chemokine Receptors
8.6.2.1 CCL2
A single-nucleotide polymorphism (SNP) in the CCL2 promoter, based on the substitution of an adenine by a guanine in position −2518 (A < −2518 < G), is associated with increased CCL2 secretion [113]. This polymorphism with an allelic frequency close to 30 % is associated with an increased susceptibility to the development of breast, gastric, and oral squamous cancer. However, it is not associated with an increased risk of developing hepatocellular and prostate cancer, glioblastoma, and melanoma. Despite this lack of association with the development of melanoma, CCL2 polymorphism is associated with increased Breslow index, suggesting its link with melanoma progression [114]. CCL2-2518G variant is also associated with increased metastases development in nasopharyngeal and breast cancer. In the former case, the deleterious effect of the polymorphism is observed only after radiotherapy [115]. Overall, the deleterious effect of the CCL2-2518G allele-associated increase of CCL2 expression is consistent with the protumoral effect of TAM in most tumors, as previously described above.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree