Summary of Key Points
- •
Robotic surgery can be used for completely portal (no utility incision) or robotic-assisted (uses utility incision) techniques.
- •
Appropriate patient and port positioning are critical for a successful performance of robotic lobectomy.
- •
Perioperative morbidity and mortality for robotic lobectomy are comparable to that for video-assisted thoracoscopic surgical (VATS) lobectomy.
- •
Robotic lobectomy may have advantages in terms of surgeon ergonomics, mediastinal lymph node dissection, and intraoperative blood loss over VATS lobectomy.
- •
Robotic lobectomy can be done safely and is being increasingly used for anatomic pulmonary resections.
Definitions
A general thoracic operation is defined as any operative procedure for lesions or structures in the thorax, including but not limited to lesions or pathology in the mediastinum, pulmonary parenchyma, chest wall muscles or skeletal structures, diaphragm, or esophagus.
A robotic system is defined as any machine or mechanical device that uses a computer to translate human movements into the movement of robotic instruments. The robotic instruments or tools, not the surgeon’s hands, interact with the patient’s tissue.
A robotic thoracic operation is defined as a general thoracic operation that is minimally invasive (i.e., no rib spreading) and in which the surgeon’s and assistant’s views of the operative field are via a monitor rather than through an incision. Moreover, the procedure utilizes a robotic system for all or mostly all of the crucial aspects of the operation. For pulmonary resection, crucial surgical aspects include dissection and ligation of the pulmonary arteries and veins, dissection and removal of the mediastinal and hilar lymph nodes, and bagging of the specimen. For mediastinal operations, dissection and removal of the mediastinal lesion are robotically performed. For esophageal operations, dissection of the esophagus and/or the esophageal lesion, resection and/or bagging of the specimen, removal of the thoracic lymph nodes, and possibly anastomosis of the esophagus to the stomach or other chosen conduit are crucial tasks completed with the robotic system.
We have suggested a nomenclature that differentiates completely portal robotic operations (CPRs) from operations in which a utility incision is used, which are referred to as robotic-assisted operation or robotic-assisted thoracic surgery. Such a nomenclature specifies the number of robotic arms implemented and is defined as follows.
A CPR is defined as an operation that uses ports only (incisions that are only as large as the size of the trocars placed in them). In this case the air in the pleural space or chest cavity does not communicate with the ambient air in the operating room, carbon dioxide is used to insufflate the chest, and the only port incision that is larger than the trocars that go through them is one through which a specimen contained in a protective bag is removed.
A robotic-assisted operation is defined as a procedure in which a utility incision is used (defined as either an incision in the chest that may or may not have trocars or robotic arms placed through it or an incision that allows communication between the ambient air in the operating room and the pleural space), which does not involve spreading of the ribs, and in which carbon dioxide insufflation is used selectively (only as needed).
The number of robotic arms used during the operation is included in the nomenclature and is separated by a hyphen after the type of operation is specified. The abbreviation for the type of operation also includes a one-letter initial to indicate the specific procedure. For example, a CPR lobectomy using four arms is a CPRL-4, and a CPR segmentectomy using three arms is a CPRS-3 ( Table 28.1 ).
| Completely Portal Robotic | Robotic Assisted | |
|---|---|---|
| Suggested abbreviations | CPR | RA |
| Designation includes the number of robotic arms used | Yes (e.g., CPRL-4, completely portal robotic lobectomy using 4 arms) | Yes (e.g., RAL-4, robotic-assisted lobectomy using 4 arms) |
| Rib spreading | No | No |
| Access or utility incision made | No | Yes |
| Carbon dioxide insufflation used | Yes | Sometimes |
| Communication between pleural space air and ambient air in operating room | No | Yes |
| Trocars placed though all incisions | Yes | No |
| Incisions bigger than size of trocars used | No | Yes |
| Site of specimen removal | Usually over the anterior aspect of the tenth rib | Usually over the anterior aspect of the fourth rib |
History of Surgical Robotics
Industrial robots are mechanical arms that, working alone or in cooperation, perform precise, complex, repetitive tasks, and manipulations under computer control. Robot arms are increasingly flexible in terms of the objects they can work on and the tasks they perform, including capabilities that require visual and other sensing systems linked to powerful computers with artificial intelligence software.
Surgical robots also consist of mechanical arms that attach to surgical instruments. However, although computers filter and scale the movements and manipulations carried out by these arms, surgeons always directly control the arms. Robotic surgical systems have been in development since the 1980s. Intuitive Surgical Inc. and Computer Motion Inc. emerged as the two main companies producing robotic systems for minimally invasive surgery in the first decade of the 21st century. Intuitive Surgical’s robot arms are controlled manually by the surgeon; Computer Motion’s system employed voice control too. Both companies obtained limited approval from the US Food and Drug Administration (FDA) for their systems. The two companies merged in 2003. Several other companies in Europe and the United States are developing robotic surgical systems: most are intended for minimally invasive surgery, but others are being developed to perform open surgery or remote surgery.
The da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) is currently the only FDA-approved robotic system for lung surgery. The surgeon sits at a console some distance from the patient, who is positioned on an operating table in close proximity to the robotic unit with its three or four robotic arms. The robotic arms incorporate remote center technology, in which a fixed point in space is defined, and the surgical arms move around it to minimize stress on the thoracic wall during manipulations. The system’s small proprietary EndoWrist instruments, which are attached to the arms, are capable of performing a wide range of high-precision movements. The surgeon’s hand movements with the so-called master instruments at the console control the EndoWrist instruments. These master instruments sense the surgeon’s hand movements and translate them electronically into scaled-down micromovements to manipulate the small surgical instruments. Hand tremor is filtered out by a 6-Hz motion filter. The surgeon observes the operating field through console binoculars. The image comes from a maneuverable high-definition stereoscopic camera (endoscope) attached to one of the robot arms. The console screen can also display digital input from electrocardiography, computed tomography, and other imaging modalities. The Firefly Fluorescence Imaging (Intuitive Surgical, Inc) involves a camera head with laser-based illuminator to visualize vascular and lymph node flow in three dimensions after injection of fluorescent dye.
The console also has foot pedals that allow the surgeon to engage and disengage different instrument arms, reposition the master controls on the console without moving the instruments themselves, and activate electric cautery. A second optional console allows tandem surgery and training.
Robotic Lobectomy: Technical Aspects
The number of robotic pulmonary resections continues to increase. The team learning curve for robotic surgery is steep; however, the learning curve for the typical thoracic surgeon may be less than that for VATS lobectomy, especially for lymph node dissection. This difference may be one reason why the popularity of robotic resection is increasing among surgeons. A review of the guidelines and pathways to team building and credentialing for robotic pulmonary resections follows.
Operating Room Configuration
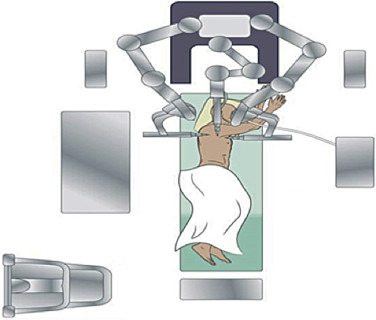
As with any operation, planning each stage of the operation is crucial to ensure success. The robot adds anxiety to inexperienced robotic surgeons and anesthesiologists. Thus planning the room layout before the operation is essential and includes positioning the bedside cart, robot, nurses’ table, monitors, and patient relative to the anesthesia equipment. The robot is driven in over the patient’s head during lobectomy; thus precise planning and communication of the position of two monitors and the distance between the operating surgeon at the console and the scrub nurse and surgical assistant(s) at the patient’s bedside are needed ( Fig. 28.1 ).
Console
The surgeon’s console should be positioned in such a way that good communication with the surgical team at the bedside can be established. The da Vinci Surgical System console contains a microphone that amplifies the voice of the surgeon to the rest of the team. The presence of a second console permits easy exchange of control between surgeon, medical student, resident, or fellow for training purposes; this second console, if used, should be located fairly close to the primary console.
Robot/Bed
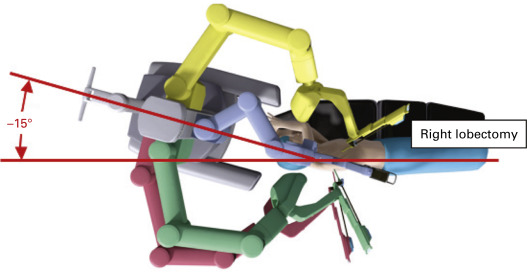
The approach of the robot to the patient’s side should be clear of any obstacles. The robot is driven over the patient’s head on a 15-degree angle to open up robotic arm 3 over the head and shoulder ( Fig. 28.2 ). In addition, monitors are positioned so that the bedside assistants and scrub nurse have a clear view.
Depending on the size of the room and the arrangement of immobile structures within it, the patient’s bed may need to be turned such that the patient’s head is located well away from the ventilator and anesthesia console. A long extension for the endotracheal tubing should be used if necessary.
When the robot is set up, robotic arm 3 should be placed on the robot side opposite the side of the lobectomy (e.g., if performing a right lobectomy, robot arm 3 should be located on the robot’s left when facing the robot).
Surgical Team
The surgical bedside assistant should be in position at the patient’s ventral side (i.e., in front of the patient’s abdomen and chest), with a monitor on the opposite side. The scrub nurse should be in position with the instrument stand near or over the patient’s feet, as in conventional thoracotomy or VATS.
Patient Positioning
The patient is placed in the supine position, general anesthesia is induced, and the patient is intubated with a left-sided double-lumen endotracheal tube. Proper placement of the double-lumen tube is assisted greatly by the use of a flexible pediatric bronchoscope and is crucial to a smooth operation because access to the patient’s head and endotracheal tube will be limited by their positioning and the presence of the robot after docking.
After the double-lumen tube is secured, the patient is placed in the lateral decubitus position with the operative side up. An axillary roll is placed. We do not use an arm board; rather, we place the patient’s back at the edge of the table, leaving space in front of the face to fold the arms and expose the axilla for port placement ( Fig. 28.2 ). We have used this positioning for more than 17 years for our thoracotomies because when a four-arm robotic approach is used, robotic arm 3 can move on a plane that is below the bed and avoid conflicts with that arm and the operative bed itself. Padding should be used around the arms and head to prevent nerve damage during the surgery. We use large foam pads to protect the patient’s head and arms. This easy, quick, and cost-effective technique requires no special equipment and is reproducible.
Port Placement/Docking
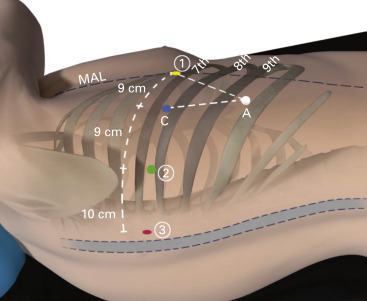
The ports are inserted in the seventh intercostal space over the top of the eighth rib for upper/middle lobectomy and in the eighth intercostal space over the top of the ninth rib for lower lobectomy ( Fig. 28.3 ).