Summary of Key Points
- •
Immunotherapy has entered a new era in lung cancer and is rapidly changing the standard of care.
- •
Vaccines as monotherapies have shown marginal efficacy in certain settings.
- •
Program death-1 (PD1) and PD1 ligand (PDL1) inhibitors have showed marked and durable responses for a broad subset of lung cancer patients with overall survival rates that far exceed what has been seen with chemotherapy. In addition, the overall toxicity rate is lower than what has been seen with chemotherapy.
- •
PDL1 is a potential but imperfect biomarker of response in nonsmall cell lung cancer.
- •
Combination therapies (with other immunomodulators, vaccines, chemotherapy, and radiation) are actively being explored to extend the benefit of PD1 inhibition to more patients.
Lung cancer is the leading cause of cancer-related death, and 85% of patients with this condition are diagnosed with nonsmall cell lung cancer (NSCLC). Modest improvements in cure rates have been observed among patients with early and locally advanced disease; nevertheless, the majority of patients die as a result of a metastatic progression of the cancer. Patients with advanced-stage NSCLC have a median overall survival (OS) of 10 months and a 1-year survival rate of about 40% when treated with modern platinum-doublet chemotherapy, although this time frame has somewhat been extended to a median OS of 13.9 months with maintenance pemetrexed following cisplatin–pemetrexed in nonsquamous lung carcinoma. Tumors with specific oncogenic drivers are particularly sensitive to specific tyrosine kinase inhibitors that represent significant improvements over chemotherapy for these subgroups. Such therapy is approved for tumors with activating epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase translocation, or c-ros oncogene (ROS) translocation, and several other oncogenic drivers have targeted therapies in development. However, there are still large populations of NSCLC without any targeted therapy available to them.
Immunotherapy using programmed death receptor (PD1) or PD1 ligand (PDL1) inhibitors has already changed the outcomes for advanced NSCLC patients, in some cases more than doubling survival times, and the survival improvements that are possible with combination therapy are still to be determined. As in many tumor types, the rapid advent of immunotherapy-based regimens built on a backbone of PD1 or PDL1 inhibition is rapidly changing our standards of care. Many trials of PD1/PDL1 inhibitors are ongoing in patients in different stages of lung cancer, in combination with vaccines, chemotherapy, radiation, targeted therapy, and with novel immunotherapeutics, suggesting that more change is yet to come.
Immunologic Dysfunction in Patients with Lung Cancer
The principle of immunosurveillance, that is, the ability of the immune system to recognize malignant cells as foreign and, possibly, to eliminate them, has long been accepted, but the many potential mechanisms of tumor evasion from immune surveillance are now beginning to be targeted with therapeutic agents.
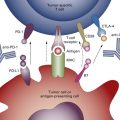
Normal immunosurveillance starts with uptake of tumor antigens by antigen-presenting cells (APCs), particularly dendritic cells ( Fig. 50.1 ). The antigens are internalized, processed into small peptide sequences, and displayed on the extracellular surface of the APC in the presence of the class I and class II major histocompatibility complex (MHC). The dendritic cell with antigenic peptides on its surface circulates to the draining lymph nodes and matures, leading to interaction with naive T lymphocytes.
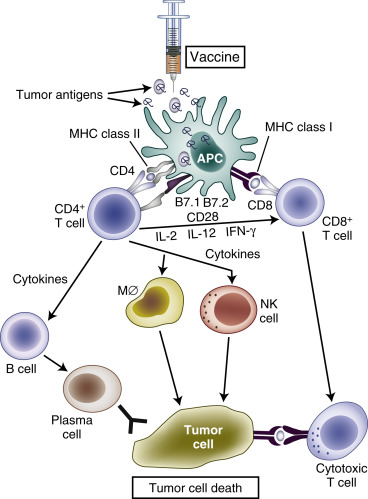
This interaction results in activation of the CD4 + T-helper lymphocytes—with liberation of several cytokines, for example, interleukin-2 (IL-2; Th1 cells), IL-12 (dendritic cells), and interferon gamma (Th1 cells)—and subsequent activation of CD8 + T cells into cytotoxic T lymphocytes. For this T-cell activation to occur, there must be an interaction between the specific T-cell receptor on naive T cells and the antigen presented by the APC on an MHC molecule. A required costimulatory event is the interaction between the B7 molecules (B7-1 [CD80], B7-2 [CD86]) on the APCs and CD28 on the T cells ( Fig. 50.2 ). Finally, activated cytotoxic T lymphocytes will recognize tumor cells (TCs) that display the complementary peptide–MHC class 1 complex on their cell surface and induce apoptotic cell death.
To prevent excessive reactions with autoimmunity and damage to normal host tissue, modulation of activated CD8 + cells is required. Activated CD8 + T cells also express cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) on their surface. Binding of CTLA-4 with CD80 or CD86 on APCs provides an inhibitory signal and limits further T-cell activation. This mechanism against autoimmunity also may be responsible for the tolerance of tumor antigens. Although PD1 was identified on exhausted T cells in 1992, it was after the discovery of the PDL1 ligand (B7-H1) on lymphoid and nonlymphoid tissues that the role of PDL1:PD1-mediated downregulation of immune activation in peripheral tissues was clarified. Interaction of PD1 with its ligand results in downregulation of T-cell mediated cell killing, altered cytokine production, and ultimately apoptosis. PDL1 is expressed in various normal tissues in response to inflammatory cytokine signaling to maintain self-tolerance. This same mechanism can be co-opted by TCs to avoid an acquired immune response to tumor-associated antigens.
Overall, lung cancer and other tumors can induce major immunologic dysfunction through a variety of mechanisms. Downregulation of antigens and decrease of expression of MHC class I molecules and costimulatory molecules lead to failure of T-cell recognition and activation. Inhibitory cytokines such as indoleamine 2,3-dioxygenase and transforming growth factor-beta (TGF-β) impede the maturation of dendritic cells and promote the development of T-regulatory cells and myeloid-derived suppressor cells, which have a powerful immunosuppressive action. Inhibition of T-cell activation by the CTLA-4 interaction with B7 and PDL1–PD1 interactions to suppress effector T-cell function can contribute centrally and locally to immune suppression. Lastly, the failure to activate apoptotic mechanisms in response to the effect of cytotoxic T lymphocytes may render the lung cancer cells insensitive to immune control.
Supporting Evidence for the Use of Immunotherapy for Lung Cancer
Although lung cancer historically was not considered to be an immunogenic malignancy, there is evidence suggesting that there may be important immune responses in patients with lung cancer. The LACE-Bio group, in a study of 1600 patients with resected early-stage NSCLC, found that marked infiltration of the tumor by lymphocytes was associated with significantly longer disease-free survival and OS (hazard ratio [HR], 0.57 [ p = 0.0002] and 0.56 [ p = 0.0003], respectively). Other studies also have shown that increased stromal infiltration of CD4 + /CD8 + T cells is independently associated with better prognosis in cases of early-stage NSCLC. By contrast, high expression of tumor-infiltrating T-regulatory cells—that is, reduced antitumor immunity—has been associated with recurrence of disease. Patients with advanced-stage NSCLC, in whom tumors have higher numbers of macrophages and CD8 + T cells compared with the surrounding stroma, have better survival rates. The overexpression of PDL1 by TCs in NSCLC has been demonstrated in several retrospective studies, which have reported rates of 27% to 58%. Several of these studies report an increased inflammatory infiltrate associated with PDL1 overexpression. The expression of PDL1 by TCs may also be mediated by the activation of specific oncogenes associated with NSCLC including EGFR. Smoking status has also been correlated with elevated PDL1 expression. However, the association between OS and PDL1 expression remains controversial with reports of both an associated improvement and decrease in OS. PDL1 overexpression and associated activation of the PD1 pathway thus appear to be broadly exploited by TCs in NSCLC as a means to evade T-cell-mediated antitumor activity. These findings support the strategy to use or manipulate the immune system to generate antitumor effects to improve the outcomes for patients with lung cancer.
The approaches toward therapeutic modulation of immune responses fall into two primary categories. The first, “active” immunotherapy includes modes of stimulating the immune response, such as ILs, interferons, or antigen-specific immunotherapy. Historically, and even in some recent large phase III trials, “active” immunomodulatory agents that stimulate immune response have been associated with disappointing results in lung cancer. Historical examples include trials investigating bacillus Calmette-Guérin, levamisole, or interferons and ILs. More recent examples include trials investigating PF-3512676 (ProMune), an agonist of the toll-like receptor 9 that enhances maturation of dendritic cells, and talactoferrin alpha, an oral recombinant human lactoferrin, acting by dendritic cell recruitment and activation in the gut-associated lymphoid tissue.
Therapeutic cancer vaccination is a mode of antigen-specific “active” immunotherapy in which the immune system is primed to produce antigen-specific antibodies, CD4 + T-helper cells, and CD8 + cytotoxic T lymphocytes against relevant tumor-associated antigens. This has been an active area of research in lung cancer, but one that has had largely marginal benefits seen as monotherapies to date, which are reviewed in the following sections.
The second approach, “passive immunotherapy,” involves blocking inhibitory signals that suppress immune responses against cancer. Examples of the latter include monoclonal antibodies that modulate T-cell activity by inhibiting CTLA-4, PD1, or PDL1, also known as immune checkpoint inhibitors. It is this latter category of therapy, specifically PD1 and PDL1 antibodies, that has galvanized the field of immunotherapy in lung cancer by demonstrating marked clinical benefit in subsets of patients and this, therefore, will constitute the majority of the discussion here.
Vaccines
Antigen-specific immunotherapeutic agents always have two major components. The first component consists of immunogenic tumor-associated antigens, which can be DNA, RNA, peptides, recombinant proteins, gangliosides, or whole TCs. However, the presence of tumor-associated antigens alone is insufficient for a therapeutic cancer vaccine as the immune system already has failed to control the cells expressing these antigens; otherwise, the tumor would not have grown to a clinical level. Therefore, a strong adjuvant is added to potentiate the immune response. This immunoadjuvant can be a phospholipid or aluminum formulation, a viral vector, a dendritic cell, or a liposome preparation. There are many vaccination strategies and compounds in development. Most have been evaluated either in the adjuvant setting or following first-line chemotherapy in the advanced setting to determine whether they can stave off progression of disease. We will review some of the agents that are still in clinical development and the change in direction now being taken with the success of immune checkpoint inhibitors.
Melanoma-Associated Antigen-A3 Vaccine
Melanoma-associated antigen (MAGE)-A3 is expressed almost exclusively on TCs and is not expressed in normal tissue, except in male germ line cells; however, such cells do not present the antigen as they lack MHC molecules. The function of MAGE-A3 is unknown, but its expression has been associated with worse prognosis in cases of lung cancer. Expression has been documented in 35% of early-stage NSCLC.
The vaccine contains a recombinant fusion protein (MAGE-A3 and protein D of Haemophilus influenzae ) in combination with an immune response-enhancing adjuvant (AS02B in the phase II study and AS15 in the phase III study).
For NSCLC, the proof-of-concept study was a double-blind, placebo-controlled randomized phase II trial. Patients with completely resected MAGE-A3-positive stage IB to stage II NSCLC were randomly assigned to receive MAGE-A3 vaccine (122 patients) or placebo (60 patients). No adjuvant chemotherapy was given, as this therapy was not recommended at the time of the study. The disease-free interval was the primary end point of the study and, at a median of 70 months after resection, there was a nonsignificant trend in favor of MAGE-A3 (HR for disease-free interval, 0.75; 95% confidence interval [CI], 0.46–1.23; p = 0.254). A potential gene signature, which was found to be predictive of the clinical activity of the MAGE-A3 vaccine in a trial involving patients with metastatic melanoma, was further validated in a separate study in early-stage NSCLC. The disease-free interval was better for actively treated patients with NSCLC and a positive gene signature compared with patients who received placebo (HR, 0.42; 95% CI, 0.17–1.03; p = 0.06); among patients with a negative gene signature, no benefit was found (HR, 1.17; 95% CI, 0.59–2.31; p = 0.65).
On the basis of these data, a large double-blind, randomized placebo-controlled phase III trial (MAGE-A3 as Adjuvant NSCLC Immunotherapy Trial [MAGRIT]) was conducted from 2007 to 2012 and reported in 2016. Patients with MAGE-A3 overexpression (4210 of 13,489 originally screened) who had completely resected stage IB to stage IIIA NSCLC and adjuvant chemotherapy as clinically indicated were randomly assigned (in a 2:1 ratio) to receive MAGE-A3 vaccine or placebo. There was no difference in disease-free survival between the two groups: median 60.5 months in the vaccinated group and 58.0 months in the placebo group (HR, 0.97; 95% CI, 0.80–1.18; p = 0.76). In the absence of any treatment effect, no gene signature predictive of response could be evaluated or validated. Further development of the MAGE-A3 vaccine in NSCLC has been stopped.
Mucinous Glycoprotein-1 Vaccines
Tecemotide (L-BLP25)
Mucinous glycoprotein-1 (MUC1) is a highly glycosylated transmembrane protein that is present in normal tissue only at the apical surface of the epithelial cell. Its exact function remains unclear, but MUC1 may promote cell growth and survival. In cancer cells, MUC1 is overexpressed with loss of polarity of expression and is underglycosylated or aberrantly glycosylated, which results in unmasking of its peptide epitopes, thus identifying a potential target for immunotherapy.
Tecemotide (L-BLP25) is a peptide vaccine based on a 25-amino-acid sequence from the MUC1 protein in a liposomal delivery system (consisting of cholesterol, dimyristoyl phosphatidylglycerol, and dipalmitoyl phosphatidylcholine), which facilitates uptake by APCs, and monophosphoryl lipid A, which is added to enhance immune stimulation.
In an open-label phase II randomized study, 171 patients with stage IIIB to stage IV NSCLC who had a response or stable disease after first-line therapy were randomly assigned to best supportive care plus tecemotide (88 patients) or best supportive care alone (83 patients). The median OS was not significantly different in patients who were treated with tecemotide, compared with those treated with best supportive care alone (13.0 months; HR, 0.739; 95% CI, 0.509–1.073; p = 0.112). In a post hoc analysis by stage, tecemotide did not provide benefit to patients with stage IV disease but it did provide some benefit to patients with stage IIIB disease who were treated with chemoradiation therapy (HR, 0.524; 95% CI, 0.261–1.052; p = 0.069).
A phase III, double-blind study was conducted in stage III patients and confirmed these findings, showing no significant survival benefit in those treated with vaccine (829 patients; median OS, 25.6 months; 95% CI, 22.5–29.2) compared with those treated with placebo (410 patients; median OS, 22.3 months; 95% CI, 19.6–25.5 months; adjusted HR for modified intention-to-treat population, 0.88; 95% CI, 0.75–1.03; p = 0.123). Again, in the subgroup of patients who received concurrent chemotherapy plus radiation (as opposed to sequential) prior to vaccination, there was a statistically significant benefit. The OS in the vaccinated group was 30.8 months (95% CI, 25.6–36.8) compared with 20.6 months (95% CI, 17.4–23.9) for those who received placebo (adjusted HR, 0.78; 95% CI, 0.64–0.95; p = 0.016). A follow-up report with longer follow-up confirmed these findings and suggested that high levels of serum MUC1 and antinuclear antibodies (ANA), correlated with a possible survival benefit (interaction p = 0.0085 and 0.0022) for tecemotide. Further development of this vaccine as a monotherapy has been stopped.
TG4010 Vaccine
The TG4010 vaccine also targets MUC1 as well as IL-2. It uses a viral vector—attenuated Ankara virus—that has been genetically modified to express the full MUC1 protein and IL-2 and exogenous IL-2 used as an immunoadjuvant to try to overcome the T-cell suppression caused by the cancer-associated MUC1 mucin. This vaccine was tested in a slightly different setting, given not as “adjuvant” therapy after chemotherapy, but in combination therapy in the metastatic setting.
In an open-label phase II randomized study, 148 untreated patients with MUC1-expressing stage IIIB to stage IV NSCLC were randomly assigned to receive up to six cycles of cisplatin and gemcitabine with or without TG4010. The 6-month rate of progression-free survival (PFS) was not statistically different between the two arms (43% vs. 35%; p = 0.13), but higher response rate was seen in the experimental arm (43% vs. 27%; p = 0.03). In a subgroup analysis, the level of activated natural killer (NK) cells acted as a possible predictive factor. In patients with normal levels of NK cells, the PFS rate at 6 months was 58%, compared with 38% for placebo ( p = 0.04), and OS was significantly better (18 vs. 11.3 months; p = 0.02). Side effects related to TG4010 were mild and most commonly included injection-site reactions, fever, and abdominal pain.
A phase IIb randomized, double-blind, placebo-controlled trial to evaluate first-line chemotherapy with or without TG4010 for patients with MUC1-positive (at least 50% expression on TCs) stage IV NSCLC was reported in 2016. In this trial, patients received four to six cycles of a platinum-based doublet chemotherapy (bevacizumab allowed) and TG4010 or placebo until disease progression or treatment discontinuation for any reason. The primary end point was PFS to validate the predictive value of the previously identified TrPAL biomarker (CD16, CD56, and CD69 triple-positive activated lymphocytes) as a marker of benefit. From 2012 to 2014, 22 patients were randomized to TG4010 and chemotherapy and 111 to placebo. The median PFS in the vaccinated group was 5.9 months versus 5.1 months in the placebo group (HR, 0.74; 95% CI, 0.55–0.98; one-sided p = 0.019). The primary end point was met because in patients with the TrPAL biomarker less than the upper limit of normal, the HR for PFS was less than 1 (HR, 0.75; 95% CI, 0.54–10.3), although notably the CI crosses 1. The phase III portion of the study is continuing with OS as the primary end point.
Belagenpumatucel-L
Belagenpumatucel-L is an allogeneic whole-tumor-cell vaccine derived from four irradiated NSCLC cell lines (two adenocarcinomas, one squamous cell carcinoma, and one large cell carcinoma) that have been transfected with a plasmid containing a TGF-β2 antisense transgene, which downregulates TGF-β2. Elevated levels of TGF-β2 are known to be linked to immunosuppression in patients with cancer, and TGF-β2 levels are inversely correlated with prognosis in patients with NSCLC.
Whole-tumor-cell vaccines can expose the host immune system to a wide range of tumor antigens. Although autologous TCs generally are thought to provide the panel of antigens most representative of the tumor in an individual patient, their practical use is limited by the complex production process. The G-VAX vaccine initially was associated with promising findings in a phase II study involving 83 patients with NSCLC, but further development was abandoned because of logistic problems.
Belagenpumatucel-L was investigated in a phase II single-arm dose-range study involving 75 patients with NSCLC of various stages that suggested improved survival among advanced-stage patients treated at high dose. This vaccine was further studied in the phase III randomized STOP trial. Patients with stage IIIA (T3 N2), stage IIIB, and stage IV disease, without progression after first-line chemotherapy, were randomly assigned to treatment with intradermal belagenpumatucel-L (270 patients) or placebo (262 patients), once monthly for 18 months and then once at 21 months and 24 months. There was no difference in survival for the vaccinated group (median OS, 20.3 months), compared with the placebo group (17.8 months; HR, 0.94; p = 0.594). There were also no differences in PFS. However, a prespecified Cox regression analysis demonstrated that time from chemotherapy (<12 weeks) and the receipt of radiation were associated with benefit, suggesting that there still may be a role for this vaccine in certain settings.
Epidermal Growth Factor Vaccine
Given the prevalence of EGFR overexpression in NSCLC and the importance of EGFR signaling in multiple subtypes of NSCLC, an EGF vaccine (CimaVax) was developed in Cuba with recombinant human EGF coupled to a carrier protein (P64K Neisseria meningitidis protein) and emulsified in Montanide ISA-51. A randomized phase III trial conducted in Cuba in 405 NSCLC patients with stage IIIB/IV disease who had completed first-line chemotherapy demonstrated a nonsignificant improvement in median survival for the vaccinated group (10.83 months, 95% CI, 8.85–12.71 months) versus the control group (8.86 months, 95% CI, 6.69–11.03 months). When a weighted log rank was used, given the late separation of curves, the survival difference became significant. High baseline EGF levels were associated with poorer survival overall, but for those with high baseline EGFR, vaccination was associated with improved survival (HR, 0.41; 95% CI, 0.25–0.67; p = 0.0001); however, the CIs for median survival in each arm were very wide. A new international randomized trial, comparing best supportive care alone with best supportive care plus this vaccine after conventional first-line treatment for advanced NSCLC, was started in 2011 and is still ongoing ( ClinicalTrials.gov identifier: NCT01444118).
Racotumomab
This compound, formerly known as 1E10, is an anti-idiotype ganglioside vaccine. Gangliosides are involved in cell–cell recognition, cell matrix adhesion, and cell differentiation and are expressed on the surface of TCs. The compound targets Neu-glycosylated sialic acid-containing ganglioside (NeuGc-GM3), a variant of the normal Neu-acetylated sialic acid ganglioside, which is identified almost exclusively in transformed cells, making NeuGc-GM3 an attractive target for immunotherapy.
Racotumomab was evaluated in a prospective, randomized, open-label study in Cuba in 176 patients with stage III/IV NSCLC with objective response or stable disease after standard first-line treatment. The intent to treat median OS was 8.2 months versus 6.8 months in the placebo arm (HR, 0.63; 95% CI, 0.46–0.87; p = 0.004), but the OS in both arms was notably low. Fewer than 30% of patients were evaluated for immune response. A confirmatory randomized phase III multinational trial is still ongoing ( ClinicalTrials.gov identifier: NCT01460472).
Overall, strategies using vaccine in early- or later-stage lung cancer have proved disappointing, with marginal improvements in survival in select circumstances that require validation. However, one explanation for the lack of effectiveness of vaccines is the possibility that local immune suppression around the tumor prevents the mounting of a sufficient immune response to actually shrink tumors and halt growth. The advent of checkpoint inhibition may alter the potential for vaccines through combination strategies as described in the following section.
Immune Checkpoint Inhibitors: Agents and Clinical Development
Far more promising than the “active immunotherapy” agents studied to date is the recent experience with “passive immunotherapy,” such as monoclonal antibodies that modulate T-cell activity by inhibiting CTLA-4, PD1, or PDL1 (immune checkpoint inhibitors).
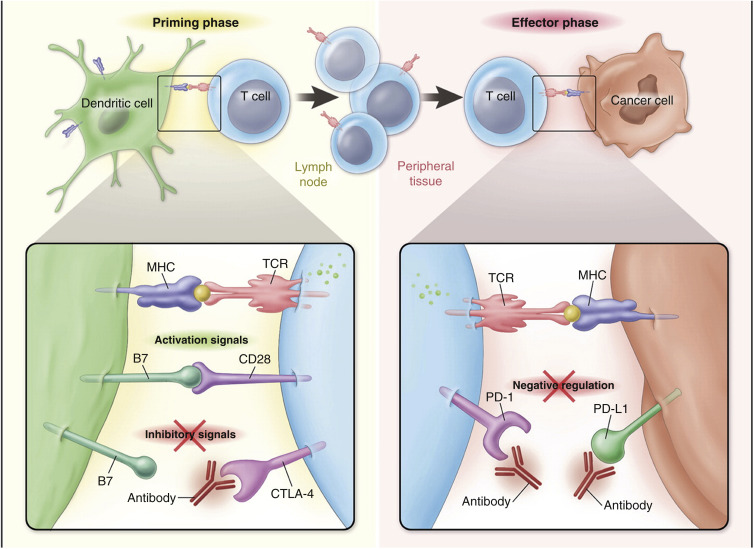
CTLA-4 is an immunomodulatory molecule that acts as a negative regulator in the early phase of T-cell-mediated immune responses. With CTLA-4, immune activation takes place when there is binding between the antigen presented on the dendritic cell and the T-cell receptor in the presence of costimulatory binding of B7 and CD28, whereas inhibition takes place when coexistent B7 binding is with CTLA-4 (see Fig. 50.2 left panel ). Anti-CTLA-4 antibodies prevent interaction between CTLA-4 and its ligands, resulting in relief of the inhibitory signal provided by CTLA-4 and consequent enhancement of activation and proliferation of tumor-specific T cells.
In the later phase, immune effector functions take place when there is binding between an antigen presented on tumor tissue and the T-cell receptor. The normal role of PD1 and its ligands is to limit the activity of T cells in peripheral tissues at the time of an inflammatory response to limit autoimmunity. In the context of antitumor activity of CD8 + lymphocytes, however, this same blockade does not allow efficient TC kill (see Fig. 50.2 right panel ). Anti-PD1 and anti-PDL1 antibodies reverse this inhibition, and thus they restore antitumor immune effector functions.
Checkpoint Inhibitors
Anti-CTLA-4 Antibodies
Ipilimumab
Ipilimumab is a humanized immunoglobulin G1 (IgG1) anti-CTLA-4 monoclonal antibody, which is an effective and approved agent for the treatment of melanoma but has had no clear single-agent activity in NSCLC or SCLC. In a phase II trial in both NSCLC and SCLC, patients received six cycles of carboplatin–paclitaxel chemotherapy plus placebo or plus ipilimumab in two different sequences: either concurrent (four cycles of chemotherapy plus ipilimumab followed by two cycles plus placebo) or phased ipilimumab (two cycles of chemotherapy plus placebo followed by four cycles plus ipilimumab). For eligible patients, ipilimumab or placebo was administered every 12 weeks as maintenance therapy after chemotherapy and the primary end point was immune-related PFS (irPFS) as defined by Wolchok and colleagues. In the NSCLC study, the irPFS was significantly better for patients treated with phased ipilimumab than for controls (HR, 0.72; p = 0.05), but it was not significantly better for patients treated with concurrent ipilimumab than it was for controls (HR, 0.81; p = 0.13, but in the small squamous cell subgroup, HR was 0.55). Median irPFS rates were 5.7 months, 5.5 months, and 4.6 months for the phased ipilimumab, concurrent ipilimumab, and control groups, respectively. There was no significant difference in OS, and the rates of grade 3 and grade 4 immune-related adverse events were high (15%, 20%, and 6%, respectively).
The results of the SCLC study were similar. Phased ipilimumab improved irPFS compared with the control (HR, 0.64; p = 0.03) but concurrent ipilimumab did not (HR, 0.75; p = 0.11). The median irPFS rates for the phased ipilimumab, concurrent ipilimumab, and control groups were 6.4 months, 5.7 months, and 5.3 months, respectively. There was no significant improvement in OS (HR, 0.75; p = 0.13). The overall rates of grade 3 and grade 4 immune-related adverse events were 17%, 21%, and 9%, respectively.
On the basis of these results, two phase III trials using phased ipilimumab were initiated with OS as the primary end point: one in squamous cell carcinoma and one in SCLC. Both studies showed absolutely no OS benefit or PFS benefit with increased rates of toxicities for the ipilimumab arm (squamous study [unpublished] and SCLC study). In the squamous cell study, among 388 patients treated with blinded therapy with chemotherapy plus ipilimumab, the median OS was 13.4 months compared with 12.4 months in 361 patients treated with chemotherapy plus placebo (HR, 0.91; 95% CI, 0.77–1.07; p = 0.25; results not published but are presented on ClinicalTrials.gov [NCT01285609]). In the SCLC study, median OS was 11 months for the ipilimumab-containing arm and 10.9 months for the placebo plus chemotherapy arm (HR, 0.94; 95% CI, 0.81–1.09; p = 0.38).
Anti-PD1 and Anti-PDL1 Antibodies
Several monoclonal antibodies are in various stages of research. We focus here on the antibodies currently approved for treatment of NSCLC, including nivolumab, pembrolizumab, and atezolizumab. Other agents, such as durvalumab and avelumab, are also in clinical development in lung cancer, although the focus for these agents has been on combination studies, which are still in the early stage and ongoing at this time and so are beyond the scope of this chapter. Table 50.1 outlines some of the major completed and ongoing studies evaluating checkpoint inhibitors as an alternative to standard therapy. Table 50.2 outlines major trials completed and ongoing combining checkpoint inhibitors with standard chemotherapy.
| Compound | Trial | Design | No. of Patients | Study Population | Treatment Arms | Primary End Point |
|---|---|---|---|---|---|---|
| Nivolumab | Phase III CheckMate 026 NCT02041533 | Randomized vs. first-line platinum-based chemotherapy | 541 | Treatment-naive metastatic NSCLC | Nivolumab vs. platinum-based chemotherapy | PFS in prespecified subset of those with ≥5% PDL1 expression |
| Pembrolizumab | Phase III (KEYNOTE-024) | Randomized vs. first-line platinum-based chemotherapy | 305 (randomized population) | Treatment-naive metastatic NSCLC with ≥50% PDL1 tumoral expression | Pembrolizumab vs. platinum-based chemotherapy | PFS: 10.3 months vs. 6.0 months for chemotherapy (HR, 0.50; 95% CI, 0.37–0.68; p < 0.001) |
| Pembrolizumab | Phase III (KEYNOTE-042) NCT02220894 | Randomized open-label | 1240 | Treatment-naive metastatic NSCLC with ≥1% PDL1 positivity | Pembrolizumab vs. platinum-based chemotherapy | OS |
| Atezolizumab | Phase III: Several ongoing studies IMpower110 NCT02409342 (others ongoing as well) | Randomized open-label | 570 | Treatment-naive metastatic NSCLC | Atezolizumab vs. platinum + pemetrexed (nonsquamous) or platinum + gemcitabine (squamous) | PFS and OS |
| Nivolumab/ipilimumab | Phase III CheckMate 227 NCT02477826 | Randomized open-label | 2220 | Treatment-naive metastatic NSCLC | Nivolumab vs. nivolumab/ipilimumab vs. platinum-based chemotherapy ± nivolumab | OS and PFS of the monotherapy vs. chemotherapy or combination vs. chemotherapy |
| Durvalumab/tremelimumab | Phase III (MYSTIC) NCT02453282 | Randomized open-label | 810 | Treatment-naive metastatic NSCLC in subgroups (PDL1 positive and PDL1 negative) | Durvalumab vs. durvalumab/tremelimumab vs. platinum-based chemotherapy | PFS and OS of the combination vs. platinum-based chemotherapy |
| Durvalumab/tremelimumab | Phase III (NEPTUNE) NCT02542293 | Randomized open-label | 800 | Treatment-naive metastatic NSCLC in stratified subgroups (PDL1 positive and PDL1 negative) | Durvalumab/tremelimumab vs. standard of care platinum-based chemotherapy | OS |
| Compound | Trial | Design | No. of Patients | Study Population | Treatment Arms | Primary End Point |
|---|---|---|---|---|---|---|
| Nivolumab (Rizvi et al. 2016) | Phase I, CheckMate 012 NCT01454102 | Open-label combination of nivolumab with platinum-based chemotherapy | 56 | Treatment-naive metastatic NSCLC | A. Nivolumab + cisplatin/gemcitabine B. Nivolumab + cisplatin/pemetrexed C. Nivolumab (10 mg/kg) + carboplatin/paclitaxel D. Nivolumab (5 mg/kg) + carboplatin + paclitaxel | Safety RR A. 33%; B. 47%; C. 47%; D. 43% 24-week PFR A. 51%; B. 71%; C. 38%; D. 51% 2-year OS A. 25%; B. 33%; C. 27%; D. 62% |
| Nivolumab | Phase III, CheckMate 227 NCT02477826 | Randomized first-line platinum-based chemotherapy ± nivolumab vs. nivolumab alone vs. nivolumab + ipilimumab | 2220 | Treatment-naive metastatic NSCLC | Platinum-based chemotherapy ± nivolumab OR nivolumab/ipilimumab OR nivolumab alone | PFS and OS of nivolumab and nivolumab/ipilimumab vs. chemotherapy alone |
| Pembrolizumab (Langer et al. 2016) | Phase II (KEYNOTE-021) NCT02039674 | Open-label, randomized phase II pembrolizumab ± cisplatin/pemetrexed | 123 | Treatment-naive metastatic nonsquamous NSCLC | Cisplatin + pemetrexed ± pembrolizumab | RR: 55% RR in combination vs. 29% with chemotherapy alone PFS: 13 months vs. 8.9 months (HR, 0.53; 95% CI, 0.31–0.91; p = 0.0102) |
| Pembrolizumab | Phase III (Merck 189, NCT02578680 and Merck 407, NCT02775435) | Randomized first-line platinum-based chemotherapy ± pembrolizumab | 570 (189) and 560 (405) | Treatment-naive metastatic NSCLC | Pembrolizumab vs. platinum–pemetrexed in nonsquamous NSCLC (189) or platinum–nab-paclitaxel in squamous NSCLC (405) | PFS |
| Atezolizumab | Phase III Several ongoing studies IMpower110 NCT02409342 (others ongoing as well) | Randomized open-label | 570 | Treatment-naive metastatic NSCLC | Platinum + pemetrexed (nonsquamous) or platinum + gemcitabine (squamous) ± atezolizumab | PFS and OS |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree