Summary of Key Points
Chest Wall:
- •
Invasion of parietal pleura and chest wall indicates T3; involvement of vertebral body indicates T4 chest wall tumor.
- •
Extensive resection required.
- •
Long-term survival possible postresection if:
- •
No distant metastases
- •
No mediastinal lymph node involvement
- •
Complete (R0) resection.
- •
- •
Systematic lymph node dissection should be performed as part of resection.
- •
Choice of prosthesis for chest wall reconstruction determined by size and location of chest wall defect.
Pancoast:
- •
Superior pulmonary sulcus: uppermost extent of costovertebral gutter.
- •
Challenging to treat due to involvement of adjacent vital structures, including brachial plexus, subclavian vessels, and spine.
- •
By definition, stage IIB or higher; mediastinal staging via endobronchial ultrasound or mediastinoscopy recommended.
- •
Induction chemoradiotherapy followed by surgical resection is the standard of care.
- •
Operative approaches:
- •
Posterior (Paulson) approach
- •
Modified posterolateral periscapular (Masaoka) approach
- •
Anterior (Dartevelle/Spaggiari) transmanubrial approach.
- •
Chest Wall Tumors
General Principles
Invasion of the parietal pleura or chest wall by a primary lung cancer is a relatively rare occurrence, reported in 5% to 8% of all cases of lung cancer. Invasion of the parietal pleura and the chest wall suggests a T3 tumor, and involvement of the vertebral bodies suggests a T4 tumor. Tumors infiltrating the second or first rib and surrounding structures usually are considered to be superior sulcus or Pancoast tumors when neurologic symptoms are present. Pancoast tumors are described in detail in the latter part of this chapter.
Extensive resection is required to remove tumors invading the chest wall. Although such tumors once were considered to have a dismal prognosis, many series have shown that long-term survival may be possible when the patient has no distant metastases, no involvement of mediastinal nodes, and evidence of complete (R0) resection as demonstrated by histologically negative margins in the ribs as well as in the muscles and soft tissues of the chest wall. In addition, a thorough lymph node evaluation by means of either systematic node dissection or at least a lobe-specific node dissection related to the location of the primary tumor is required. A minimum of six lymph node stations must be removed, of which three must be located in the mediastinum and must include the subcarinal station. According to the definition of complete resection as proposed by Rami-Porta et al. there must be no extracapsular extension and the highest mediastinal lymph node must be negative. Complete (R0) resection can be challenging in cases of posterior tumors located near the costovertebral angle or involving vertebral bodies, and analysis of frozen sections is not feasible for tumors with osseous margins.
Staging
The goal of surgical treatment in cases of T3 and T4 lung cancers is to obtain an R0 resection. Surgical treatment may be part of a multimodality approach that includes induction chemotherapy or chemoradiation therapy to reduce the tumor volume and to optimize resection margins. A thorough preoperative evaluation is necessary. Functional operability depends on a detailed cardiopulmonary assessment as outlined by a working group of the ERS together with the ESTS. T3 and T4 tumors require at least a lobectomy, but no specific criteria have been developed to determine whether a patient will tolerate a planned chest wall resection. Nevertheless, as it is clear that a chest wall resection may induce additional respiratory compromise, clinical judgment by an experienced thoracic surgeon and discussion of each individual case by a multidisciplinary tumor board are necessary when this procedure is anticipated. Published series report that mediastinal nodal involvement is a poor prognostic factor and that extensive surgery is not warranted when mediastinal lymph node metastases are present.
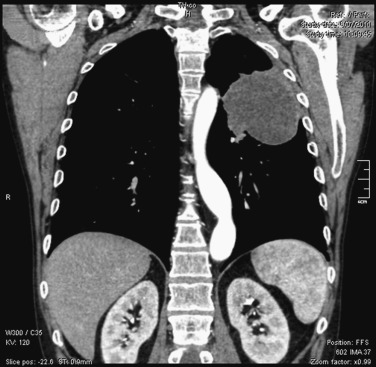
Computed tomography (CT) of the chest with use of intravenous contrast medium is the preferred method for defining the extent of the primary tumor and evaluating the involvement of hilar and mediastinal lymph nodes ( Fig. 30.1 ). Findings on CT images that are used to identify osseous or soft-tissue chest wall invasion include obliteration of the extrapleural fat plane, the length of tumor contact with the pleural surface, the angle of the tumor with the pleura, and clear evidence of chest wall invasion. A combination of several criteria increases sensitivity. For paravertebral and superior sulcus tumors, magnetic resonance imaging (MRI) of the chest is required to determine neural or vertebral involvement. Respiratory dynamic MRI has been shown to have a sensitivity of 100% and a specificity of 83% for determining chest wall invasion but has not been widely adopted. Ultrasonography of the chest wall also may be helpful but does not show superior sulcus tumors. Positron emission tomography (PET), preferably, integrated PET–CT, should be performed for every patient to evaluate local–regional extension and possible distant spread precluding surgical intervention.
The pathologic status of the mediastinal nodes should be confirmed before a large chest wall resection is planned. Endobronchial ultrasound and endoscopic ultrasound with transbronchial or transesophageal biopsy are currently the procedures of choice. In selected cases, these procedures are supplemented with mediastinoscopy to reduce the false-negative rate as much as possible.
Surgical Resection
Depending on the location of the primary tumor and its extension into the chest wall, the incision is carefully chosen and may be centered on the anterior, lateral, or posterior chest wall. The thoracic cavity is entered away from the primary tumor, as every attempt should be made to obtain an en bloc resection with complete removal of the primary tumor together with the invaded chest wall to avoid any spillage of tumor cells in the pleural cavity. Video-assisted thoracoscopic surgery may be helpful for initial evaluation.
The precise margins to be obtained around the primary tumor have not been exactly determined, but most authors have agreed that at least 1 cm is required. Once the pleural space has been entered, chest wall involvement is evaluated and a determination is made as to whether an extrapleural resection or a full-thickness chest wall resection is required. Stoelben and Ludwig described four categories of chest wall involvement for determining the subsequent resection ( Table 30.1 ). When the tumor easily detaches from the chest wall in the extrapleural plane by finger dissection, this usually indicates only inflammatory adhesions that do not require chest wall resection. Frozen section analysis of suspicious areas of the parietal pleura may clarify this. When the tumor is densely adherent or frankly invades the chest wall, rib resection is indicated. For anterior tumors, resection of part or all of the sternum may be needed. For posterior tumors, resection of transverse processes or vertebral bodies may be needed, and a spine surgeon (either an orthopedic surgeon or a neurosurgeon) should work collaboratively with the thoracic surgeon. However, resection of these vertebral structures is infrequently seen outside the classic Pancoast (superior sulcus) position. To reduce trauma to the uninvolved extrathoracic muscles, rib resection can be performed from inside the chest with use of the technique described by Cerfolio et al.
| Intraoperative Findings | T Status b | Procedure |
|---|---|---|
| Lung and tumor not fixed to chest wall | Not T3 | Standard resection |
| Inflammatory adhesions between tumor and parietal pleura or previous inflammatory pleuritic | Not T3 | Extrapleural resection |
| Tumor has penetrated visceral pleura to the parietal pleura | T3 | Extrapleural lobectomy probably possible |
| Tumor has infiltrated soft-tissue or osseous chest wall | T3 or T4 | Lung and chest wall resection |
a Adapted from Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg. 2009;35(3):450–456.
Lobectomy or bilobectomy is the procedure of choice for pulmonary resection. A pneumonectomy is necessary in some cases, but it is a high-risk procedure when combined with extensive chest wall resection and should be performed only in centers with extensive experience.
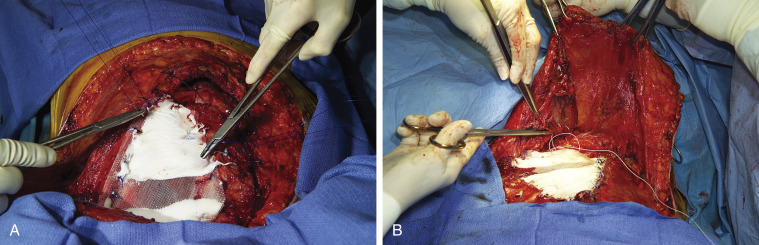
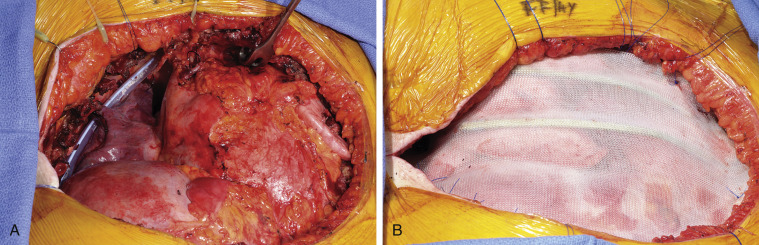
Multiple techniques are available for reconstruction of the chest wall. No reconstruction is required for defects of 3 cm or less that are covered by the scapula. However, when the defect is located at the tip of the scapula, chest wall reconstruction is required to prevent entrapment of the scapula, which is a highly symptomatic complication that is associated with poor cosmetic results. Polypropylene and polyglactin meshes, polytetrafluoroethylene patches, and the so-called Marlex mesh with methylmethacrylate (MMM) sandwich technique are options for the reconstruction of large defects. Close cooperation with a plastic surgeon is required in situations in which soft-tissue reconstruction is needed to cover a chest wall prosthesis. Polytetrafluoroethylene is frequently used as the standard material. However, for large anterior or anterolateral defects, the MMM sandwich technique provides greater immediate chest wall stability ( Figs. 30.2A and 30.2B ) with the lowest risk of postoperative respiratory insufficiency. A new moldable system composed of titanium with connecting bars and rib clips is useful for obtaining a rigid basis when reconstructing the chest wall. This system is particularly useful for large defects associated with skin ulceration and infection, for which the MMM sandwich technique is contraindicated ( Fig. 30.3A–B ). Synthetic material should be covered by viable muscle or musculocutaneous flaps to reduce the risk of infection.
Results and Long-Term Survival
At experienced centers, the mortality and morbidity rates associated with these procedures have decreased. The mean postoperative mortality rate is about 6%, with the majority of deaths being caused by pulmonary complications and respiratory failure. This finding underlines the importance of careful preoperative cardiorespiratory evaluation and discussion within a multidisciplinary team before the decision is made to proceed with an extensive resection. In addition to standard complications after thoracotomy, specific complications related to the chest wall resection include infection necessitating removal of the prosthetic material, herniation of the scapula, paradoxical respiration (flail chest), and, in cases involving dissection close to the spinal cord, paraplegia and leakage of cerebrospinal fluid.
In selected cases, induction chemotherapy or chemoradiation therapy may be helpful for decreasing the tumor volume and limiting the subsequent chest wall resection, but such therapy has not been standard treatment except for Pancoast tumors. Adjuvant chemotherapy or radiotherapy also remains controversial, and no specific guidelines exist for patients who have chest wall resection. Radiotherapy may be indicated for patients with close or positive surgical margins, but no randomized evidence is available to support its routine use.
In most large series, the 5-year overall survival rate has been approximately 30% to 40%. Long-term survival depends on lymph node involvement and the completeness of resection. In 1999, in a large series of 334 chest wall resections, Downey et al. showed that the 5-year survival rate was 32% for patients with apparently complete (R0) resections but only 4% for those with incomplete (R1 and R2) resections. These findings were confirmed in later studies. In a large series of 531 patients with pT3 lung cancer, a relatively uniform prognosis was found for the different subgroups of T3 involvement. For the 407 patients with chest wall involvement, the 5-year survival rate was 43%. In a comparative study involving the seventh edition of the tumor, node, metastasis classification system, the prognosis for 140 patients with T3 chest wall invasion was not significantly different from that for 28 patients with tumors measuring more than 7 cm in size without chest wall invasion. In a multivariate analysis of 107 patients who had chest wall resection for the management of lung cancer invading the chest wall, the completeness of resection, tumor size, node status, depth of invasion, and completeness of adjuvant chemotherapy were independent prognostic factors. In that series, the overall 5-year survival rate was 26%.
With regard to the extent of pulmonary resection, a recent series showed that pneumonectomy combined with chest wall resection is feasible for highly selected patients. In that series of 34 patients, the mortality rate was 2.9%, the morbidity rate was 38%, and the overall 5-year survival rate was 47%. For patients with N0, N1, and N2 disease, the 5-year survival rates were 60%, 56%, and 17%, respectively. After complete (R0) resection, the rate of local recurrence is very low. N2 involvement can be considered to be a marker for systemic disease, and most patients with N2 involvement will die of distant metastases.
Quality of life after chest wall resection is important to consider, but published data are sparse. In a retrospective series of 51 patients who were treated with chest wall resection, quality of life was only moderately impaired. Subjective parameters, including dyspnea, correlated well with quality of life, whereas objective measurements of pulmonary function did not.
Pancoast Tumors
Historical Background
Nonsmall cell lung cancer (NSCLC) of the superior sulcus, commonly termed Pancoast tumors, is challenging to treat because of its involvement of adjacent vital structures, including the brachial plexus, subclavian vessels, and spine. Originally described by a radiologist, Henry Pancoast, in 1932, Pancoast tumors were thought to be uniformly fatal until the 1950s, when, on the basis of anecdotal experience, induction radiotherapy and resection were found to be curative. During the next 40 years, this approach remained the standard of care, with advances limited to the development of novel surgical techniques for T4 tumors involving the subclavian vessels and spine. However, complete resection was usually achieved in only 60% of patients, and the overall 5-year survival rate remained at about 30%, indicating a need for novel therapy. During the 1990s, concurrent cisplatin-based chemotherapy and radiotherapy followed by resection was shown to be safe and effective for the management of some stage III NSCLC. The findings of small studies suggested that this treatment might be appropriate for Pancoast tumors, which led to a large North American trial, the results of which established the use of induction chemoradiation therapy followed by resection as the standard of care. Other studies subsequently corroborated the results of the North American trial (see Table 30.2 ).
| Author (y) | No. of Patients | Induction Therapy | Rate of Complete Resection (R0) (%) | 5-Year Overall Survival Rate (%) |
|---|---|---|---|---|
| Marra et al. (2007) | 31 | Cisplatin + etoposide + 45 Gy | 94 | 46 |
| de Perrot (2008) | 44 | Cisplatin + etoposide + 45 Gy | 89 | 59 |
| Pourel et al. (2008) | 107 | Cisplatin + etoposide + 45 Gy | 90 | 40 |
| Kunitoh et al. (JCOG 9806) (2008) | 76 | Mitomycin, vinblastine, and cisplatin + 45 Gy (split course) | 89 | 56 |
Anatomic Definition
The original definition of a Pancoast tumor was a carcinoma of uncertain origin, arising in the extreme apex of the chest, that was associated with shoulder and arm pain, atrophy of the hand muscles, and Horner syndrome. Anatomically, the pulmonary sulcus is synonymous with the costovertebral gutter, which extends from the first rib to the diaphragm. The term superior pulmonary sulcus is used to describe the uppermost extent of this recess. Unbeknown to Pancoast, the most accurate description of this type of tumor was reported in 1932 by Tobias, who recognized it as a peripheral lung cancer.
This original definition has been expanded to include tumors that do not involve the brachial plexus or stellate ganglion. Tumors involving the chest wall at the level of the second rib or below do not meet the criteria for classification as Pancoast tumors. Chest wall involvement may be limited to invasion of the parietal pleura in the superior sulcus but typically extends to involve the upper ribs, vertebral bodies, subclavian vessels, nerve roots of the brachial plexus, or stellate ganglion.
The thoracic inlet can be divided into three compartments on the basis of the insertions of the anterior and middle scalene muscles on the first rib and the posterior scalene muscle on the second rib. The anterior compartment, located anterior to the anterior scalene muscle, contains the subclavian and internal jugular veins and the sternocleidomastoid and omohyoid muscles. The middle compartment, located between the anterior and middle scalene muscles, includes the subclavian artery, the trunks of the brachial plexus, and the phrenic nerve. The posterior compartment contains the nerve roots of the brachial plexus, the stellate ganglion, and the vertebral column.
Originally, Pancoast tumors were considered to be located only posteriorly. However, they also may be located anteriorly, with predominantly vascular involvement rather than neurologic or vertebral involvement. Surgeons should be adept at both anterior and posterior approaches, as a combined procedure may be necessary to obtain a complete resection.
Pretreatment Evaluation
The presence of a Pancoast syndrome is not always associated with NSCLC. Other diseases, including lymphoma, tuberculosis, and primary chest wall tumors, may be associated with an apical mass and chest wall involvement. Transthoracic needle biopsy should be performed to establish a diagnosis before treatment.
Pancoast tumors are, by definition, classified as stage IIB or higher and require an extent-of-disease evaluation before treatment is initiated, including contrast-enhanced CT of the chest and upper abdomen, whole-body PET, and MRI of the brain ( Fig. 30.4A–B ). Because Pancoast tumors with mediastinal node metastases (N2 or N3 disease) have a poor prognosis, mediastinal staging via either endobronchial ultrasound or mediastinoscopy should be considered.
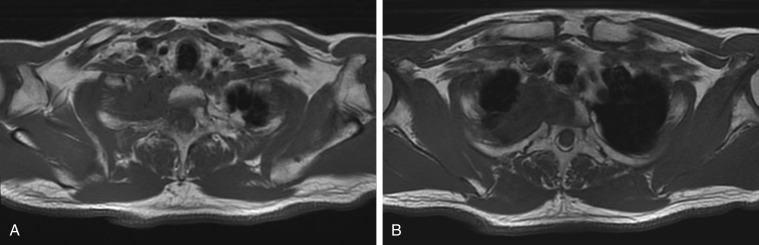
MRI with use of intravenous contrast medium is the modality of choice for evaluating structures of the thoracic inlet, including the brachial plexus, subclavian vessels, spine, and neural foramina and is crucial for preoperative planning. The extent of nerve root involvement must be assessed. Resection of the T1 nerve root usually does not cause motor function deficit, but resection of the C8 nerve root or lower trunk of the brachial plexus leads to loss of hand and arm function. A careful neurologic examination is informative and supplements MRI findings. Pain extending along the ulnar aspect of the forearm and hand is consistent with T1 involvement. Weakness of the intrinsic muscles of the hand indicates involvement of the C8 nerve root or lower trunk of the brachial plexus. Resection of a Pancoast tumor should be planned jointly with a spine neurosurgeon to allow optimal patient selection and the best chance of complete resection.
Patients must be evaluated to determine whether they can tolerate combined-modality therapy. Performance status, renal function, and neurologic function must be adequate in order for the patient to receive platinum-based chemotherapy. Pulmonary function tests and, when necessary, cardiac stress tests are done to evaluate the ability of the patient to tolerate pulmonary resection.
Multimodality Treatment
The management of NSCLC of the superior sulcus during the past 70 years can be classified into four eras. Pancoast first described these tumors as “a peculiar neoplastic entity found in the upper portion of the pulmonary sulcus of the thorax . . . evidently epithelial in its histopathology, but its exact origin is uncertain.” During the ensuing 20 years, these tumors became recognized as NSCLC but were considered inoperable and incurable. In 1956, Chardack and MacCallum reported the case of a patient in whom a poorly differentiated squamous cell carcinoma was managed with en bloc resection of the right upper lobe, involved chest wall, and nerve roots, followed by adjuvant radiotherapy (65.28 Gy over 54 days). The patient was alive and disease-free 5 years later. In 1956, Shaw reported on a patient with the typical Pancoast syndrome who was referred for palliative radiotherapy. After treatment with 3000 cGy of radiation, the pain resolved and the tumor decreased in size, so Shaw performed a radical resection similar to that described by Chardack and MacCallum. The complete resection and long-term survival that were achieved in that case prompted Shaw et al. to test this treatment strategy in additional patients. In 1961, they reported excellent local control and longer-than-anticipated survival in a study of 18 patients who were treated with 3000 to 3500 cGy of radiation over 2 weeks, followed by complete en bloc resection of the involved lobe, chest wall, and nerve roots 1 month later.
After that report, induction radiation (3000 cGy in 10 fractions over 2 weeks) and en bloc resection via an extended posterolateral thoracotomy became the standard of care for superior sulcus NSCLC. For 30 years (the second era in the management of Pancoast tumors), the basic therapeutic strategy for these tumors remained unchanged. The findings of multiple series ( Table 30.3 ) confirmed the original results reported by Shaw and Paulson but also identified adverse prognostic factors, including the presence of mediastinal node metastases (N2 disease), involvement of the spine or subclavian vessels (T4 disease), and incomplete (R1 or R2) surgical resection. The largest published series, which included 225 patients who were treated at Memorial Sloan-Kettering Cancer Center between 1974 and 1988, confirmed the importance of these prognostic factors. Although the operative mortality rate was low (4%), R0 resection was achieved for only 64% of T3 N0 tumors and 39% of T4 N0 tumors and local–regional recurrence was common. The survival rate after lobectomy was better than that after limited pulmonary resection, and the addition of intraoperative brachytherapy to resection did not appear to improve the survival rate. The overall 5-year survival rate was 46% for T3 N0 tumors, 13% for T4 N0 tumors, and 0% for N2 disease. These results emphasized the need for new treatment strategies to improve both local control and overall survival.