GENERAL PRINCIPLES
Cancer and its treatments are a major cause for impairments and disability. Because cancer treatments have become increasingly successful and have improved survival, there has been an increasing focus on quality of life and, in particular, rehabilitation. Cancer rehabilitation is practiced in outpatient clinics, oncology wards, inpatient rehabilitation units, skilled nursing facilities, nursing homes, long-term acute care centers, palliative care units, and hospices. Common diagnoses addressed include asthenia, deconditioning, hemiplegia, spinal cord injury, peripheral neuropathy, somatic and neuropathic pain, steroid myopathy, lymphedema, bowel/bladder management, limb amputation, and limb dysfunction.
The major goal of cancer rehabilitation is to improve quality of life by minimizing the disability caused by cancer and its treatments and decreasing the “burden of care” needed by patients with cancer and their caregivers. The more patients can do for themselves, the more personal dignity they are able to maintain and the less help they require from those around them.
In 1978, Justus Lehmann, supported by the National Cancer Institute (NCI), screened 805 randomly selected patients with cancer, identifying multiple problems in the population of patients with cancer who are amenable to rehabilitation interventions along with barriers limiting the delivery of rehabilitation care. More than 30 years later, many of Lehmann’s remediable cancer rehabilitation problems and barriers to rehabilitation care remain the same (Table 59-1).
| Remediable Rehabilitation Problems | Barriers to Delivery of Rehabilitation Care | |
|---|---|---|
| Psychological/psychiatric impairments | Lymphedema management | Lack of identification of patient problems |
| Generalized weakness | Musculoskeletal difficulties | Lack of appropriate referral by physicians unfamiliar with the concept of rehabilitation |
| Impairments in activities of daily living | Swallowing dysfunction | Patient too ill to participate |
| Pain | Impaired communication | Patient denies need |
| Impaired gait/ambulation | Skin management | Cancer prognosis too limited |
| Disposition/housing issues | Vocational assessments | Rehabilitation unavailable |
| Neurologic impairments | Impaired nutrition | No financial resources |
| Vocational assessments | Lymphedema management | |
| Impaired nutrition |
These problems are familiar to rehabilitation professionals because many are also found in traditional noncancer rehabilitation patients. Lehman also described major barriers to the delivery of cancer rehabilitation care, including the lack of identification of these problems by oncologists and the lack of referral to rehab professionals for a rehabilitation intervention. In addition, there are multiple patient-related factors that can affect the successful rehabilitation of the patient with cancer. Several reported by DeLisa include reduced life expectancy, extensive comorbidities, degree of pain, the dynamic nature of cancer lesions, the demands of anticancer therapies, and the desire to spend remaining time with loved ones (1).
In 1980, Dietz categorized cancer rehabilitation into four stages: preventive, restorative, supportive, and palliative (2). Preventive rehabilitation occurs before or immediately after a treatment to prevent loss of function or disability. An example would include preamputation stump care teaching and ambulation with a walker in a patient with a lower extremity sarcoma. In 2001, Courneya described a concept called “buffering” whereby a patient with cancer undergoes exercises and therapies to increase the patient’s physical and functional reserves before cancer treatment (3).
Restorative rehabilitation occurs in patients who are believed to be disease free or will have an anticipated relatively stable disease course. Taking our previous example, a patient with lower extremity sarcoma with no known metastatic disease following amputation undergoes prosthetic rehabilitation. These first two stages are not significantly different from conventional nononcologic rehabilitation. Fortunately, as survivorship has increased, restorative rehabilitation has become more prominent in addressing issues in this population, including disability, return to work, and lymphedema management.
If patients are unable to achieve or maintain a state of remission, supportive rehabilitation is performed to sustain function and provide symptom management. Expanding on our example, a patient with metastatic sarcoma may be provided therapies and durable medical equipment (DME) to promote functional independence while receiving chemoradiation.
Unfortunately, patients may succumb to their malignancy, or its accompanying morbidities, and efforts to maximize function may require a transition to focusing on quality of life. Palliative rehabilitation is provided to reduce discomfort and improve independence in patients with advanced disease. Our example patient with sarcoma and advancing metastatic disease may have failed multiple treatment regimens and now requires rehabilitation to return home with some degree of assistance. The emphasis of palliative rehabilitation is typically to get the patient home safely as soon as possible. The patient likely has limited time to live and the costs of a lengthy inpatient rehabilitation stay must be taken into consideration. Goals once the the patient is home in a safe environment focus on family and transfer training. Higher-level goals should be addressed as an outpatient or through home health therapy.
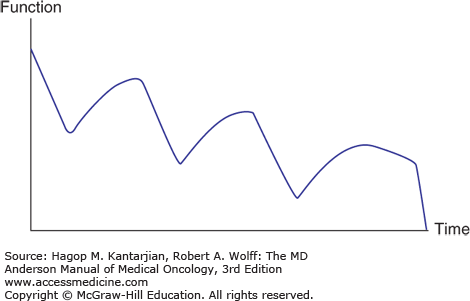
The last two stages described by Dietz (1) are relatively unique to cancer rehabilitation. The typical course for a patient in conventional rehabilitation (eg, after a stroke) is continued improvement after the inciting event. In the patient with cancer with persistent disease, however, the war continues with brief victories followed by declines as the disease progresses (3) (Fig. 59-1).
Multiple studies have established a need for rehabilitation in the population of patients with cancer (4,5). Functional improvements of patients in cancer rehabilitation have been demonstrated in a number of settings, including inpatient (6,7,8,9) and palliative care (10,11), on a consultation basis (12), in a hospice setting (13,14), and for outpatient settings (15). The maintenance of physical activity and exercise has been implicated in increasing survival, with the greatest quantity of evidence in patients with breast and colon cancer. Multiple mechanisms may exist, and some have proposed these findings to be related to levels of insulin/c-peptide, along with the positive effects of buffering on physiological reserves, potentially allowing for more treatment (16,17). Cancer physiatrists must also address medical sequelae and complications that are unique to this patient population. With limited training in general medical and surgical fields, these comorbidities may serve as a significant challenge and require frequent communication between the primary oncology and rehabilitation teams. The transfer rate from inpatient rehabilitation to acute care teams is high compared to other rehabilitation diagnoses (18).
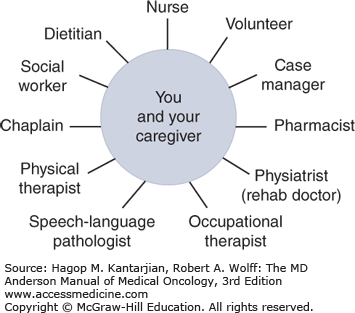
Rehabilitation goals are accomplished by the efforts of a comprehensive interdisciplinary team of health-care professionals, including the rehabilitation physician, rehabilitation nurse, physical therapist, occupational therapist, speech therapist, dietitian, pharmacist, chaplain, social worker, and case manager. Each member of the team has specific expertise in assisting the patient with a care plan of maximizing medical stability, function, financial resources, and caregiver involvement for a discharge that is as safe and meaningful as possible (Fig. 59-2).
At the University of Texas MD Anderson Cancer Center (MDACC), our cancer rehabilitation practice includes seven physical medicine and rehabilitation physicians and a rehabilitation therapy staff of over 100 physical therapy and occupational therapy clinicians. Rehabilitation therapists see over 300 inpatients and 100 outpatients per day. Patients include those with most of the different tumor types seen in the institution, the most common being brain, spine, lung, breast, hematologic, genitourinary, gastrointestinal, and head and neck tumors. The most common inpatient rehabilitation diagnoses included asthenia, gait abnormality, dyspnea, hemiparesis, spinal cord injury, and neurogenic bowel and bladder. Common outpatient rehabilitation diagnoses include lymphedema, myofascial pain, rotator cuff dysfunction, peripheral neuropathy, and low back pain. Inpatient and outpatient electromyograms are performed for neuropathic and myopathic diagnoses and spasticity management.
When describing the functional status of a patient with cancer, the Karnofsky Performance Scale is often used. It is the most widely used scale both clinically and in research in oncology patients (19). It is an easy and quick generalized measurement of a patient’s function. Weaknesses of the scale include overgeneralization (unable to measure specific tasks) and poor correlation with cognition (20).
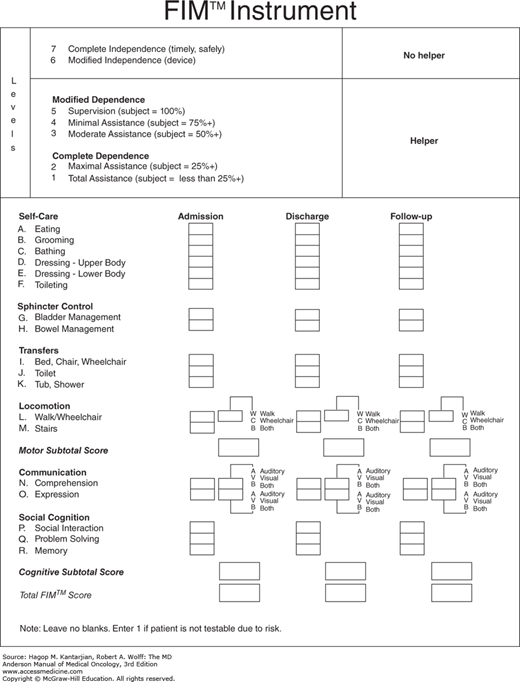
In rehabilitation, the outcome scale most often used is the Functional Independence Measure (Fig. 59-3). This multidimensional scale addresses 18 items from a scale of 1 (total assistance) to 7 (complete independence). Items are subdivided into self-care, sphincter control, transfers, locomotion, communication, and social cognition. An aggregate score is also often useful out of a total of 126. Criticisms of the scale include that it is too general and omits items that are important for specific populations, such as those with spinal cord injuries (21).
PRACTICAL ASPECTS OF CANCER REHABILITATION
Physiatry is a holistic specialty that accounts for medical needs while focusing on a patient’s functional issues. Safety is a primary concern of rehabilitation health-care professionals. The first question a physiatrist often asks is, What is the minimum level of safe function for this patient to be discharged? That “safe” functional level goal depends on the patient’s current functional level, strength, cognition, amount of supervision or assistance available on discharge, accessibility of home conditions, and financial resources. Physiatrists must ask patients and their caregivers detailed social questions.
Common obstacles confronted are a lack of assistance or supervision at home. This often is due to the patient’s significant other being required to work during the day, the significant other’s inability either physically or mentally (eg, dementia) to care for the patient, or having no one available to care for them.
One of the most practical and simple rehabilitation techniques that an inpatient must learn is to transfer. A transfer is a change in station or position from sitting in bed to standing or from sitting in bed to sitting in a chair. A person must transfer to get into a wheelchair or into a car seat. Patients cannot effectively mobilize until this is accomplished. Depending on the patient’s level of disability, a transfer may be performed to sit to stand, to stand and pivot, with a sliding board, or with a lift requiring total assistance. After basic transfers are mastered, ambulation may be the next goal to increase mobility. Weakness from paresis, deconditioning, neuropathy, or brain injury can also make self-care difficult. Practical skills such as feeding, grooming, bathing, and dressing are taught or relearned to improve independence.
ACUTE CANCER REHABILITATION
Rehabilitation begins when a patient is admitted into the hospital. Impairments are identified, and based on the practical considerations mentioned previously, realistic and suitable rehabilitation goals are set. If a patient is medically stable with unmet functional and safety goals, the patient’s activity tolerance and performance trajectory are used to guide determination of the appropriate intensity and location for additional rehabilitation.
REHABILITATION OF INDIVIDUALS WITH BRAIN TUMORS
Primary brain tumors are less than 2% of all malignancies but are the second leading cause of death from neurologic disorders after stroke (22). One-half to two-thirds of intracranial tumors have been reported to be primary tumors (23). Primary brain tumors are classified by cell of origin; the primary system of classification is that of the World Health Organization (WHO), which divides tumors into nine categories. The most common categories include tumors that displace brain parenchyma of the intracranial supratentorial compartment. Of these tumors, the most common in adults are the astrocytomas, in particular grade IV astrocytoma, otherwise known as glioblastoma multiforme (GBM) (24). The median survival for a patient with GBM has been reported between 7 and 17 months (25).
In addition to primary brain tumors, brain metastases are estimated to occur in 20% to 40% of patients with cancer. The most common mechanism of metastasis to the brain is through hematogenous spread. Most of the metastases are located in the cerebral hemispheres, followed by the cerebellum and then the brainstem. The incidence of brain tumor metastases is rising, possibly due to the increasing length of survival of a patient with cancer (26), increasing ability to diagnose a tumor with improved radiographic imaging (27), and possibly recent chemotherapy agents, which may weaken the blood-brain barrier (28).
Normal brain parenchyma can be destroyed or compressed by the tumor, and the location of the tumor determines the resultant neurologic deficit. Surgical resection may exacerbate these deficits by creating inflammation or peritumoral infarct (29). Radiation treatment has long been an integral part of brain tumor treatments and often results in collateral damage. Early acute radiation leukoencephalopathy is likely due to increased cerebral edema. Late delayed radiation reactions include focal cerebral radiation necrosis, diffuse cerebral radiation injury (DCRI), and combined-therapy diffuse white matter injury/leukoencephalopathy. Clinical DCRI has been reported in 2% to 5% of patients with metastases and 19% of 1-year survivors after whole-brain radiation (30,31).
The most common neurologic deficits include impaired cognition (80%), weakness (78%), visual-perceptual deficits (53%), sensory loss (38%), and bowel/bladder dysfunction (37%). Other deficits include cranial nerve palsy, dysarthria, dysphagia, aphasia, ataxia, and diplopia, which are less common. Approximately 75% of patients with a brain tumor have three or more neurologic deficits concurrently, and 39% have five or more deficits (32). Because of the diverse nature of these neurologic deficits, comprehensive multidisciplinary inpatient rehabilitation is often necessary for these patients. In rehabilitation medicine, the physical impairments that could result in functional deficits are primarily addressed.
Patients who have impairments resulting in functional decline that could affect bed mobility, ambulation, transferring from sitting or lying to a standing position, or activities of daily living (ADLs) (eg, eating, grooming, dressing, bathing, and toileting) can benefit from comprehensive inpatient rehabilitation. Comprehensive cancer rehabilitation services are not widely available for these patients (33). Because of this, many patients with a brain tumor receive their rehabilitation at general rehabilitation facilities alongside patients with stroke and traumatic brain injury. Patients with a brain tumor have similar efficiencies of improvement when compared to traumatic brain injury, stroke, and between brain tumor types. Lengths of stay tend to be shorter among patients with a brain tumor, possibly secondary to a need to return the patients home sooner given their shorter life expectancies (34,35,36,37,38). However, some notable differences between these populations should be noted (eg, physiatrists may need to be cognizant of the continued decline of patients with progressive tumors).
Motor impairment can be due to hemiparesis, ataxia, and apraxia. Motor impairment may lead to an unsafe gait pattern creating a higher risk for falls and a need for inpatient rehabilitation transfer. In inpatient rehabilitation, the patient will be seen by physical and occupational therapists. Physical therapy would focus on gait and transfers. To address transfers, efforts could be focused on sliding board transfer or stand pivot transfers. With respect to mobility, physical therapists focus on wheelchair mobility and gait with or without an assistive device (eg, a single-point cane, quad cane, rolling walker, hemiwalker). Occupational therapy would focus on problems with ADLs. Commonly addressed basic ADLs include dressing, bathing, toileting, grooming, eating, and the like. The occupational therapist and physical therapist are also aware of the cognitive component necessary for mobility and ADLs. Once a patient is functionally safe using an assistive device such as a rolling walker, he or she can be discharged home. Then, the patient can continue with outpatient rehabilitation, gradually improving his or her ambulation with an assistive device and further strengthening weakened muscles by way of a progressive resistance exercise program.
The pattern of recovery of muscle strength and function does not always follow the pattern of recovery observed in patients with stroke. However, the stroke recovery pattern is often used as a guideline for patients with brain tumors. The recovery of strength occurs in a proximal-to-distal direction, with flaccidity and decreased muscle tone progressing to spasticity and increased muscle tone. The spasticity in the affected limbs can evolve into flexor or extensor synergy patterns. Recovery of muscle movement may plateau at any stage or may progress to isolated coordinated volitional motor movement (39,40).
Several techniques and exercises are used for neuromuscular facilitation in patients with stroke. Often, a combination of procedures and techniques from the various programs are used in patients with cancer with neuromuscular weakness. Proprioceptive neuromuscular facilitation developed by Kabat, Knott, and Voss relies on several mechanisms, such as spiral diagonal movement patterns of the extremities and quick stretch. Brunnstrom movement therapy facilitates the use of the synergy patterns mentioned as a means of developing voluntary control. Rood proposed that cutaneous sensory stimulation in the form of superficial stroking, tapping, brushing, vibrating, or icing provides facilitatory or inhibitory inputs (41).
In addition to the traditional range-of-motion and strengthening exercises as well as neuromuscular facilitation techniques, functional electrical stimulation can also be incorporated into the rehabilitation program for neuromuscular weakness. It uses a low-level electrical current that stimulates motor nerves or reflex sensory nerves to produce muscle contraction. The goal of functional electric stimulation is to produce purposeful, functional movements in paretic or paralytic muscles (42).
Sometimes, owing to weakness of the ankle dorsiflexors, it is necessary to use an ankle-foot orthosis (AFO) to improve hemiparetic gait. There are two major types of AFOs: the double metal upright AFO attached to an orthopedic shoe and the molded plastic AFO, which is more commonly used. With the plastic AFO, the footplate sits within the shoe and extends upward behind the calf. The advantages of a plastic AFO over a double metal upright AFO include better cosmesis, lighter weight, and the freedom to wear different shoes.
Shoulder subluxation, predominantly inferior, which is caused by the loss of normal motor control of the shoulder stabilizers, including the deltoid and supraspinatus muscle, is often seen in the hemiparetic patient (43). It can often be the cause of shoulder pain in hemiplegic patients (44,45). Other possible causes of shoulder pain in this patient population include complex regional pain syndrome, traction injury of the brachial plexus, rotator cuff tendinitis or tear, subacromial or subdeltoid bursitis, adhesive capsulitis, or heterotopic ossification. Diagnosis of glenohumeral subluxation is made through physical examination and radiographic evaluation. The acromiohumeral interval is compared on each side with the arms in an unsupported position during physical examination, and radiographic evaluation is used to quantify the amount of subluxation. Radiographic studies can provide an early evaluation for subluxation with slight gapping of the superior aspect of the glenohumeral joint (46).
Treatment of hemiparetic shoulder subluxation involves proper positioning of the arm, physical modalities, and exercise. The use of an arm sling can help maintain proper positioning and posture during ambulation. However, this is discouraged when the patient is seated, and its overuse may contribute to compromise of superficial blood flow as well as to joint contracture. Arm troughs and lapboards are used while patients are seated (47). Other interventions include biofeedback and functional electrical stimulation.
Hemisensory deficit and homonymous hemianopsia may be seen with hemiparesis. Visual or somatic hemineglect is more frequently seen when the nondominant cerebral hemisphere is affected. Hemispatial neglect has a negative effect on sitting balance, visual perception, wheelchair mobility safety awareness, and risk of falling (42). Patients with neglect have difficulty with hygiene and self-care activities on the affected side. Rehabilitation programs must address the issue of hemispatial neglect through focused measures led by speech therapists, occupational therapists, and physical therapists. Family training and education are important in this setting as well.
Cerebellar ataxia may be seen with mass effect within the posterior fossa. Of note, cerebellar ataxia can also be seen in paraneoplastic cerebellar degeneration and with high-dose administration of cytarabine (ara-C) or 5-fluorouracil (5-FU) (48,49). Involvement of the cerebellum can produce intention tremors, dysmetria, and dysdiadochokinesis as patients lose the ability to coordinate the agonist and antagonist muscle groups (50). The response to pharmaceutical management has been poor; consequently, physical and occupational therapy has been the mainstay of treatment for ataxia. This includes the teaching of compensatory techniques for performing basic self-care and occupational activities and the possible use of weighted bracelets or similar devices to help decrease the oscillations. Physical therapy directed at gait training with the use of assistive devices can help improve mobility in ataxic individuals (51).
Depending on its location, a tumor may be associated with deficits in speech, which can vary in severity and type. Often, one can diagnose the type of aphasia from a comprehensive neurologic examination, including speech comprehension, fluency, and repetition. These include Broca’s aphasia, Wernicke’s aphasia, anomic aphasia, global aphasia, conduction aphasia, and the transcortical motor and sensory aphasias.
A speech pathologist will implement treatment approaches, including melodic intonation therapy, Amer-Ind Code treatment, functional communication treatment, stimulation approach, and Promoting Aphasics’ Communication Effectiveness (PACE) therapy (52).
Cognitive deficits often are more problematic than motor deficits. They can arise from direct injury to the brain tissue due to the tumor itself, from surgical resection, radiation, chemotherapy, depression/anxiety, as well as medications, particularly steroids and anticonvulsants (28). Impairments most commonly seen involve limited memory and attention, decreased initiation, and psychomotor retardation (53).
The rehabilitation physician will assess the patient’s cognitive status as part of the physical examination. This assessment is needed to formulate a rehabilitation program involving speech pathologists. Specific deficits in language and cognition can further be delineated through specific testing performed by a speech pathologist. However, it is sometimes necessary to have formal neuropsychological testing done, especially if the patient wishes to return to work.
A disruption in the swallowing process can also occur in patients with brain tumors or following craniotomies. It is important to determine, through clinical assessment, whether dysphagia is present because there is the potential for serious complications, such as malnutrition and aspiration pneumonia, if dysphagia remains undetected. Often, its presence can be established from a history and neurologic examination. If dysphagia is suspected, a speech pathologist is consulted; then, daily swallowing therapy and exercises are incorporated into the therapeutic milieu.
Treatments include dietary modifications and dysphagia exercise and facilitation techniques (54). Depending on the results from a clinical swallowing evaluation or videofluoroscopic evaluation, food can be modified to different consistencies, including puree, semisolid, or solid. Liquids may also have to be thickened using various thickening (55).
Exercises and facilitation techniques are employed to aid and strengthen various components of the swallowing process. These include exercises employed for treatment for the lips to facilitate the ability to prevent food or liquid from leaking out of the oral cavity. There are exercises to assist the pharyngeal swallow by improving tongue base retraction. Vocal cord adduction exercises are instituted to strengthen weak cords to prevent aspiration.
Compensatory strategies include proper head and trunk positioning, which for most patients is to be seated upright with head midline, trunk erect, and the neck slightly flexed forward. Other techniques include the chin-tuck method and head turning and tilting during swallowing.
After dysphagia has been identified and measures are implemented for its treatment, regular follow-up to assess for improvement is required. This again can be done through clinical examination or radiographically. If improvement is noted, the diet may be advanced appropriately.
Spasticity is defined as velocity-dependent resistance to passive movement across a joint. It is an abnormality involving increased muscle tone and is one of the positive findings of the upper motor neuron syndrome. Spasticity must be distinguished from soft tissue contractures. Soft tissue contractures result from scar tissue formation and may be the result of a number of causes, including uncontrolled spasticity.
Often, brain tumors can cause muscle spasticity. This can affect the gait pattern or ADLs and, in severe cases, can cause pain and joint contractures as well as being a detriment to hygiene of the involved areas. Sometimes, spasticity may be beneficial, as when a patient may use knee extensor spasticity to assist in transferring from a sitting to a standing position. Indications for the treatment of spasticity include the need to decrease pain, improve hygiene, improve gait and transfers, minimize contractures, and improve self-care.
Treatment measures for spasticity include physical and medical interventions. Proper positioning, passive range-of-motion exercises, serial casting, splints, and braces are some of the physical interventions used in treating spasticity. Oral medications may also be used, including tizanidine, dantrolene sodium, and baclofen. Because these medications work systemically, the most common limiting side effects are excessive drowsiness and cognitive changes. Tizanidine or dantrolene is recommended by most clinicians for treating spasticity stemming from primary brain pathology (55). Often, because of the cognitive side effects of these oral medications, botulinum toxin injections, phenol injections, or intrathecal baclofen pumps may be useful. These medications act locally but are harder to administer and more invasive.
As in patients with stroke, bladder incontinence may be present in patients with brain tumors. The causes of bladder incontinence can be multifactorial and include an untreated urinary tract infection, inability to ambulate to the bathroom, and altered cognitive status. If the pontine micturition center is preserved, patients with brain tumors can have upper motor neuron bladder dysfunction, which is characterized by bladder hyperreflexia with reflex or urge incontinence and complete emptying (56). Postvoid residual volumes are generally low in the absence of bladder outlet obstruction. Persistent areflexia and retention may occur with bilateral lesions (57).
Treatment first involves identifying the cause of the bladder dysfunction. Obtaining a urinalysis with cultures and sensitivities and then starting appropriate antibiotics is the treatment for urinary tract infections. Using a bedside commode or a urinal is of benefit for patients who have weakness or inability to safely ambulate to the bathroom. A timed voiding program that has the patient urinate at set times throughout the day, before the bladder can contract, can be of help for patients with hyperreflexic urgency. Anticholinergic medications such as oxybutynin (Ditropan) or tolterodine tartrate (Detrol) can be used for persistent incontinence in this setting of a hyperreflexic detrusor (57). If the patient’s blood pressure can tolerate it, a trial of an alpha-adrenergic agent (eg, tamsulosin, terazosin) may be useful in reducing urinary resistance in older male patients who are experiencing symptoms of urinary retention.
LYMPHEDEMA
Physiatrists are often asked to assist with the care of patients with lymphedema. Malignancy (including breast, melanoma, gynecologic, lymphoma, and urologic cancers) is the number one cause of secondary lymphedema in the United States. The lymphedema can be caused by a combination of the cancer, surgical treatments, and radiation treatments. Breast cancer is the leading cause of upper extremity lymphedema in the United States and develops in 2% to 40% of patients after surgery, radiation, or both (58,59).
The majority of lymphedema cases are diagnosed clinically. The differential diagnosis would include deep venous thrombosis (DVT), venous insufficiency, myxedema, lipedema, heart failure, kidney failure, and hypoproteinemia. In difficult-to-diagnose cases, lymphoscintigraphy is the gold standard.
The treatment of lymphedema typically starts with conservative treatments consisting of manual lymph drainage, compression sleeves, and sometimes pneumatic compression sleeves. Over one or two sessions by lymphedema-trained physical therapists, patients are taught a lymphedema regimen to do at home. Measurements are often taken, and the patients are followed up periodically to ensure that they are completing the regimen as prescribed and to measure changes in their lymphedema. Progression of lymphedema can be measured using a number of techniques, including volumetric/circumferential measurements, bioelectric impedance, tonometry, and perometry. In patients with severe or difficult-to-treat lymphedema, a number of surgical options are available, including microsurgery, liposuction, and debulking procedures.
REHABILITATION OF CANCER-RELATED SPINAL CORD INJURY
Spinal cord injury in the patient with cancer has several etiologies. These involve primary spinal cord tumor or metastatic lesions. Primary tumors such as meningiomas, neurofibromas, and gliomas are relatively rare, and the majority of tumors involving the spinal cord are metastatic. The metastatic lesions that cause nerve root or spinal cord compression can be paravertebral, extradural, intradural, or intramedullary; however, 95% of metastatic lesions are extradural. These lesions most often originate from primary tumors of the breast, lung, and prostate. Other tumors that metastasize to the spine include renal, melanoma, myeloma, and thyroid. Most extradural metastases arise from the vertebral body and result in compression of the anterior spinal cord. Approximately 70% of spinal metastases occur in the thoracic spine, which has a smaller ratio of canal-to-cord diameter than the other two spinal segments (60).
Pain worse at night and in the supine position is a common clinical presentation. Weakness and sensory loss, along with the development of bowel or bladder incontinence, may indicate spinal cord compromise. Rapid progression of paraparesis over only a few hours indicates arterial compromise by tumor invasion or pressure; slowly evolving symptoms suggest gradual cord impingement and may respond to steroids and radiotherapy (61).
Corticosteroids can alleviate pain and improve neurologic function, and radiation therapy is the treatment of choice with most cases of cord compression. If the tumor involves two or three columns of the spine, spinal stability is of concern; consequently, treatment is aimed toward stabilization of the spinal column. This can be done with cervical orthoses. Sternal occipital mandibular immobilization is well tolerated and provides adequate flexion and extension as well as stability to the lower cervical segments. Philadelphia collars provide stability in flexion and extension for higher levels but do not restrict rotation and lateral bending in the lower cervical segments. The “clamshell” thoracic lumbar-spinal orthosis is used to provide thoracic and lumbar support but may not be an option in patients with friable or intolerant skin following chemotherapy or steroid use. Therefore, the Taylor-Knight brace, which limits spinal extension, and the Jewitt brace, which limits spinal flexion, can be used to provide thoracic and lumbar support (62).
Surgery is also indicated sometimes with instability and neurologic compromise; indications include pathologic fracture and dislocation, failure of radiation therapy, and rapidly progressing myelopathic signs and symptoms. Surgical stabilization can frequently alleviate the need for external bracing, which is an added benefit.
Once spinal stabilization is achieved, comprehensive inpatient rehabilitation can address the impairments, functional limitations, and disabilities associated with spinal cord compression and injury due to cancer. Individuals with nontraumatic spinal cord injuries can achieve significant gains in functional independence measurements during inpatient rehabilitation (10). It has also been suggested that due to the limited prognosis of patients with cancer with spinal cord compression, an expedited inpatient rehabilitation stay with the focus on family training and home safety should be emphasized.
For all patients with cancer with neurologic involvement and those with profound deconditioning, it is prudent to check postvoid residuals for signs of bladder dysfunction. This can be performed noninvasively by an ultrasound-mediated bladder scanner or, more accurately, by straight catheterization and measurement postvoid. If the postvoid volumes are 100 to 150 mL or greater, an intermittent catheterization program is initiated.
Tumors involving the spinal cord cause suprasacral neurogenic bladder problems, which typically result in a hyperreflexic detrusor; this is characterized by low urinary volumes, high bladder pressures, and diminished bladder compliance. Incomplete lesions may produce the supraspinal pattern, with urgency and adequate emptying, while patients with complete lesions have reflex incontinence and incomplete voiding due to detrusor-sphincter dyssynergia (57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree