REGULATION OF POLYPEPTIDE HORMONE SYNTHESIS
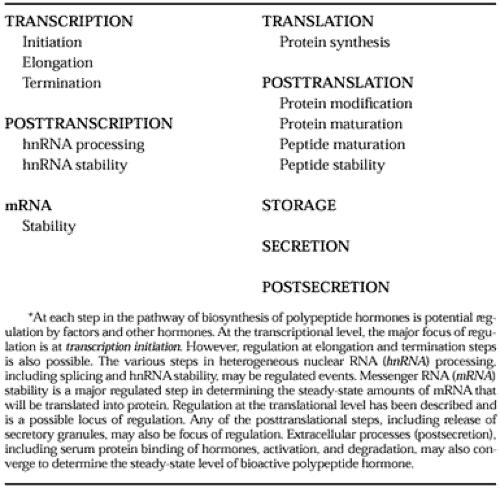
Regulation of the biosynthesis of polypeptides may occur at any of the biosynthetic levels in the pathway (Table 3-2 and Fig. 3-4). Of major interest is the regulation of peptide hormone synthesis at the transcriptional level.
Studies using gene transfer and structure-function analysis have established that specific DNA elements in the regulatory region of the transcriptional unit are critical for determining transcriptional rates of various structural regions.63,64 In particular, hormone-regulatory elements (HREs) have been characterized for glucocorticoid, estrogen, androgen progesterone, vitamin D, mineralocorticoid, retinoic acid, and thyroid hormone receptors. In each case, a DNA element 8 to 20 nucleotides long may be necessary and sufficient for conferring hormonal regulation to its associated structural region. Several factors, including the steroid and thyroid hormones, interact with nuclear receptor proteins, which interact with DNA elements directly to modulate gene transcription.65,66,67 and 68 For the glucocorticoid receptor, the glucocorticoid ligand binds to the inactive glucocorticoid receptor in the cytoplasm, present in a complex with heat shock proteins, hsp 90 and hsp 70, and others.
The activated receptor-ligand complex interacts as a transacting factor to bind the DNA element corresponding to the glucocorticoid regulatory element (GRE). Studies have been performed on GREs in genes for mouse mammary tumor virus (MMTV) and murine sarcoma viruses, human metallothionein IIa, tyrosine aminotransferase, tryptophan oxygenase, and growth hormone, and in other genes. The long terminal repeat region of MMTV contains five GREs.64,69 A consensus sequence for the putative GRE is shown by the sequence 5′-GGTA-CANNNTGTTCT-3′, inwhich N = A, C, G, or T.
The structures of the steroid and thyroid hormone receptors are better known. These hormone receptors are encoded by genes related to a viral oncogene, v-erbA.70,71 The thyroid hormone receptor is encoded by the protooncogene c-erbA. Each receptor contains a stereotypic structure, including a protein that is ˜45 to 60 kDa, with a central DNA-binding domain and a carboxyl-terminal ligand-binding domain. These and other regions mediate trans-activation, dimerization, and nuclear localization. The DNA-binding region consists of multiple cysteine and histidine residues that are critical for the formation of Zn2+ fingers first described in the DNA-binding protein TFIIIa, a transcription regulatory factor for the 5S ribosomal gene in Xenopus.72 This Zn2+finger interaction is a common motif for the binding of many eukaryotic proteins to DNA.73,74 and 75
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree