Halsted advocated radical mastectomy (RM) to achieve locoregional control for breast cancer at the end of the 19th century, stemming from his theory of the sequential progression of breast cancer metastases from the primary tumor to regional lymphatics and on to distant sites.1 The RM and extended radical mastectomy (ERM) embodied the theory of aggressive local control, and emerged as mainstays of surgical treatment for breast cancer (Fig. 68-1). Nonetheless, as early as 1912, Murphy and other proponents of pectoralis muscle preservation began to challenge these techniques with modified radical mastectomy (MRM) and total mastectomy. They demonstrated adequate local control without the associated cosmetic and functional morbidities.2 Patey and Dyson later modified the Halsted technique for resection of small (T1 and T2) breast cancers, advocating level I, II, and III axillary dissection, preserving pectoralis major, and removing only the pectoralis minor muscle.3,4 Neoadjuvant chemotherapy further served to lessen the surgical approach required by greatly facilitating resectability. The criteria for breast cancer inoperability published by Haagensen and Stout were established before the wide acceptance and advances in chemotherapy and modern radiation techniques.5 Accordingly, criteria for the role of surgery in locally advanced breast cancer (LABC) are evolving, yet still remain largely reserved for palliation, comfort, hygiene, and wound management.6
Figure 68-1
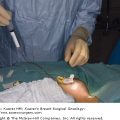
Halsted radical mastectomy. From initial description of the surgical procedure as reported in 1894. (Reproduced with permission from Halsted W. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June 1889 to January 1894. Ann Surg. 1894;5:497-555.)
The Halsted RM involves removal of all breast tissue, the pectoralis major and minor muscles, and level I, II, and III axillary and supraclavicular lymph node dissections. Since Haagensen and Stout’s 1943 publication detailing the bleak results (5-year local recurrence and survival rates of 46% and 6%, respectively) achieved with RM as sole treatment for LABC, other studies emerged comparing less aggressive surgical approaches and radiotherapy, alone and in combination with surgery, to RM for the treatment of LABC.5 Baker and associates compared the results of MRM to RM in patients with operable breast cancer, citing no statistically significant differences in 5-year survival or incidence of local or regional recurrence between the 2 surgical modalities.7 Patients with stage III disease, however, demonstrated statistically significant higher incidences of axillary and chest wall recurrences when treated with MRM versus RM, leaving the authors to conclude that MRM is appropriate for early-stage breast cancer only. Likewise, MRM sparing the pectoralis major muscle using the Patey technique was demonstrated to yield as many axillary lymph nodes as RM. This suggested that MRM was comparable to RM and sufficient for locoregional control and prognostic determination for early-stage breast cancer.8
While surgical therapy appears imperative to effective management of LABC, RM does not appear to improve outcome. Neither therapeutic doses of chest wall radiation alone9,10 or in conjunction with radical surgery11,12 yielded vast improvements in control of disease. Publication of the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04—a prospective study detailing 25-year follow-up data for primary breast cancer patients randomized to treatment with RM, total mastectomy, or total mastectomy with adjuvant radiation—revealed no benefit for RM over less radical surgical treatments.13 Indications for RM were becoming increasingly select. The application of neoadjuvant chemotherapy to patients with LABC in the decade that followed demonstrated that it in fact enhanced surgical resectability, making more radical surgery for breast cancer of historical interest only.14-16
Radical mastectomy, along with radiotherapy and chemotherapy, remains a key component of the multimodal approach to some locally advanced (T3/T4 tumors and N2/N3 disease) and recurrent breast cancers. At present time, optimal control of LABC is achieved through neoadjuvant chemotherapy, followed by surgery and radiotherapy. Surgical treatment of the intact primary stage IV breast cancer is recommended for palliation—specifically, in cases of hemorrhage, ulceration, infection, and for local wound management. Data are emerging in support of radical surgical resection of the intact primary tumor as part of a multimodal approach for patients with stage IV breast cancer and stable metastatic disease.17-20 Clinical studies to date suggest that resection of the intact primary may yield a survival advantage, yet the magnitude of this advantage in relation to morbidity remains to be determined in a prospective randomized control trial.
The technique for RM is comparable to that of MRM. RM is traditionally performed via an obliquely oriented, elliptical incision beginning at the lateral border of the sternum that is directed toward the axilla. Most critically, RM must include en bloc resection of all breast parenchyma, the nipple-areolar complex, pectoralis major and minor, and complete en bloc axillary lymphadenectomy (levels I, II, and III). This is performed with a wide skin margin that encompasses the entire skin envelope and beyond to ensure complete tumor extirpation. The design of the skin incision should incorporate previous biopsy scars and the primary neoplasm en bloc with margins that are at least 3 to 4 cm from the superior and inferior edges of the tumor. The resultant extent of dissection results in an increased morbidity compared to MRM with minimally or poor overall aesthetic result, and often significant issues in wound breakdown and healing.
The ERM evolved to include internal mammary (IM) node dissection based upon the Halstedian principle that more extensive surgical extirpation is warranted to ensure eradication of the primary tumor. Prompted by increasing evidence of frequent IM node involvement in breast cancer, the suggestion the IM nodal involvement was associated with a poorer prognosis, and retrospective comparisons suggesting improved survival from ERM, a multinational randomized trial comparing Halsted mastectomy versus ERM was initiated in 1963.21 Overall results were first reported in 1976 and updated in 1983 and showed that ERM did not improve overall survival.22 This trial, however, was underpowered and computed tomography (CT) scans were not used to stage these patients. In addition, systemic therapy was not used, further reducing the power of this study to detect a potentially clinically significant survival difference from improved locoregional control. Subsequent updates separately by the French and Italian cohorts continued to show no survival improvement with ERM for the overall cohort.23-25 Later studies concluded that ERM versus Halsted RM or total mastectomy with postoperative radiation yielded no survival advantage.25,26
The incidence of isolated metastases to IM lymph nodes (that is, without concomitant axillary nodal metastases) has been demonstrated to be quite low.27 Studies of ERM specimens have been used in attempts to identify those patients where IM nodal biopsy may be indicated, not for IM node dissection, but rather to identify those patients who may benefit from adjuvant chemotherapy and radiation to the IM nodal chain.28 Improved surgical techniques over the last few years have induced thoracic surgeons to more frequently perform sternal resections even in apparently extreme situations. These aggressive resections are not without morbidity. Prospective study evaluating quality of life of patients and risk-benefit analysis with sternal involvement and the morbidity associated with radiation-induced osteoradionecrosis of the chest wall has led surgical oncologists to adopt a more conservative approach to these cases.
Extended radical mastectomy involves a MRM or RM with complete (levels I, II, and III) axillary lymphadenectomy in addition to IM node dissection. The IM dissection is performed en bloc and includes IM vessels and lymph nodes from the first to the fourth intercostal space, with resection of the corresponding portion of the pleura. This often involves a subtotal, if not total, sternectomy to gain access to the IM nodes and varying degrees of resection of adjacent ribs.
Sternal involvement may occur either from direct invasion by enlarged IM lymph nodes or from hematogenous spread, presenting as a sternal metastasis. In patients with breast cancer, the presence of either sternal involvement or an isolated sternal metastasis is relatively uncommon, with reported incidences of 5.2% and 1.9% to 2.4%, respectively.29 In contrast to vertebral lesions, which tend to result in multicentric bony disease from spread through the paravertebral (Batson) plexus, some sternal lesions have been observed to remain solitary and localized to the sternum with time, likely due to the absence of a well-developed vascular network around the sternum.30 This microenvironment is quite different from that created by the paravertebral venous plexus and may explain why the resection of a single metastatic lesion in the sternum can potentially be curative. The limited vascular supply also explains the relatively high rate of osteoarthritis and nonhealing sternal wounds, a consideration in reconstructive approaches. Sternectomy for isolated breast cancer recurrence remains a controversial issue, with retrospective case series composing the bulk of the literature. When performed in conjunction with parasternal and mediastinal lymphadenectomy, sternectomy along with node dissection has been shown to provide additional prognostic information before commencing endocrine or chemotherapy.30 Surgery has been mainly used with palliative intent after the failure of radiotherapy, but improvement in survival following sternectomy for isolated breast cancer recurrence has been reported.31
An isolated sternal metastasis should be approached with caution, as it is more likely to herald systemic disease than to be truly solitary. A full metastatic workup is mandated, as is a multimodality treatment approach. Surgical resection should be reserved for palliation or for instances in which the other treatment modalities are not possible.
Preoperative MRI or bone scintigraphy should be reviewed to assess for involvement of the manubrium. Likewise, intraoperative specimen analysis should be conducted to assess for microscopic invasion of tumor beyond the sternomanubrial joint.
The surgical resection begins with a vertical elliptical incision centered on the mass. Mobilization is then begun first on one side of the sternum, with exposure and resection of the adjacent ribs. The sternum is approached from the periphery, reserving any critical point of bone attachment to the heart and great vessels for the final steps of the operation. Both IM vessels are identified, dissected out, and preserved (if possible) or ligated before sternal division. A bone saw is then used to transect the sternum at its upper free margin.
Bony resection margins are controversial, many authors recommend leaving 3 to 4 cm of free tissue around the tumor or the irradiated tissue,32 while others advocate removing at least 4 cm of free tissue en bloc with the resection specimen throughout the thickness of the involved bone (manubrium, body, or the entire sternum) and the anterior tracts of the bilaterally corresponding ribs, as well as 1 uninvolved rib above and below the lesion, including the related intercostal space.33-35
Partial (subtotal) sternectomy is advocated in cases of tumors localized to the sternal manubrium, inferior sternal tract, or involving the lateral portion of the bone. If partial sternectomy is deemed feasible, a surgical margin of at least 2 cm should be preserved with radical resection including a 3- to 4-cm margin macroscopically free of disease at both the cutaneous and deep-tissue levels.32 If involved with tumor, underlying lung and mediastinal structures are removed en bloc. Total sternectomy is advised in cases of manubrial, mediosternal or sternomanubrial joint involvement. In both cases, the IM veins and arteries should be spared when possible for targets in free or pedicled tissue reconstruction.
Locoregional recurrence or metastatic involvement of the brachial plexus is a condition that is often associated with LABC. In the last 25 years, safer and more refined plastic surgical approaches have been developed that provide options to treat even complex recurrent disease. Known as metastatic plexopathy, tumor infiltration of surrounding nerves is often a disabling accompaniment of LABC and may involve any of the peripheral nerve plexuses. Brachial plexopathy most commonly occurs in carcinoma of the breast and lung and is typically is associated with severe unrelenting pain as the cardinal clinical feature. Weakness and focal sensory disturbances occur in the distribution of plexus involvement. Surgical exploration and neurolysis should be performed as soon as possible after the appearance of neurologic deficits to halt the development of neural ischemia and degeneration.
Treatment is palliative and includes radiotherapy to the tumor mass and chemotherapy. In selected patients, however, subtotal surgical resection of the tumor may be warranted, with a team consisting of a surgical oncologist, neurosurgeon, and plastic surgeon. At the time of surgery, if the tumor is found to encroach upon the brachial plexus, sharp dissection should be carried out with a scalpel, as electrocautery can cause electrical stimulation of the brachial plexus and its motor branches to the shoulder and upper extremity.
Brachial plexus syndromes in patients with breast cancer can ensue from various mechanisms including metastatic involvement, radiation injury, soft-tissue changes due to surgery or radiation, ischemia, idiopathic causes, or any combination of these.36,37 Clinical history, neurologic examination, EMG, and imaging studies aid in both diagnosis and management. Thorough neurologic examination during assessment of patients with LABC is essential to rule out central nervous system involvement (brain or spinal cord metastases) and tailor adjuvant radiotherapy and chemotherapy accordingly. Likewise, neurotoxic effects from chemotherapeutic agents should be assessed frequently. The response to therapy is modest and generally short lived. Significant radiation-induced brachial plexopathy is often seen for 1 to 2 years post treatment. This is often severe, progressive, and defies surgical treatment. Efforts should be made to provide adequate pain control, to maximize remaining neurologic function, and to prevent complications of immobility produced by neuromuscular dysfunction.
Lesions of the subclavian artery can be managed surgically in a number of ways including carotid-brachial bypass, subclavian-brachial bypass, axillary-brachial bypass, and direct transthoracic revascularization with aorto-innominate bypass. The relative safety and efficacy of extrathoracic repair of brachiocephalic and subclavian occlusive disease was confirmed in early studies by Debakey and Crawford.38 When there is dense sclerosis of the axillary fossa, tunneling a vascular graft through this fibrotic tissue may be hazardous to both the axillary vein and the brachial plexus. This may also lead to an increased rate of graft failure caused by compression from scar tissue. Recent studies have emerged detailing success with retrohumeral tunneling of a reversed vein graft from the right common carotid artery to the right brachial artery to avoid the previously operated and irradiated field.39 Prior studies have revealed 2 basic principles of upper extremity revascularization: the more proximal the bypass is located, the greater the long-term patency, and the use of the common carotid artery as a donor vessel is safe.40
Choice of optimal conduit remains somewhat controversial, with excellent long-term patency rates achieved for both expanded polytetrafluoroethylene (PTFE) and reversed greater saphenous vein grafts in the proximal brachial position.41 Optimal conduit choice is a question that requires future prospective consideration. The issue of graft compression also requires constant patient awareness and minor lifestyle changes, such as wearing a strapless brassiere and carrying one’s handbag on the contralateral shoulder.
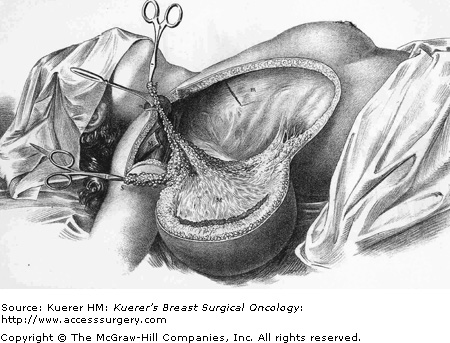
Postoperative morbidity and mortality associated with chest wall resection have been reported to range from 8% to 27% in various series.30 Stability and dynamic elasticity of the thorax are required to support the mechanics of normal respiratory function. Extirpation of a locally advanced or recurrent breast cancer often involves resection of ribs, sternum, adjacent chest wall musculature, lung, pericardium, and thymus. Chest wall reconstruction thus often requires recreation of both skeletal and soft-tissue components. Reconstruction following simple or MRM involves both immediate and delayed reconstructive options; reconstruction following RM or ERM, however, presents unique challenges, as the aims are not only aesthetic but functional. When skin graft is utilized for closure following a RM for LABC, the appearance of the graft is unaesthetic and less stable compared with more complex reconstructive methods (Fig. 68-2). Respiratory disturbance after resection of the anterior chest wall is a major problem, and different techniques of chest wall reconstruction have been described. A reconstructed chest wall should prevent paradoxical movement of the thorax, protect underlying mediastinal structures, and be immunologically inert as well as translucent on chest radiograph. The anatomic site and size of the defect must also be taken into consideration when choosing the materials and technique. Figure 68-3 describes the reconstructive ladder of increasingly complex approaches that can be applied to the reconstruction of chest wall defects.
Figure 68-2
Postoperative appearance of skin graft utilized for closure of radical defect. Locally advanced breast cancer in high-risk patients with multiple medical comorbidities as managed with a skin graft. The unaesthetic appearance of a graft as well as the less stable nature of the grafts can be noted.
Figure 68-3
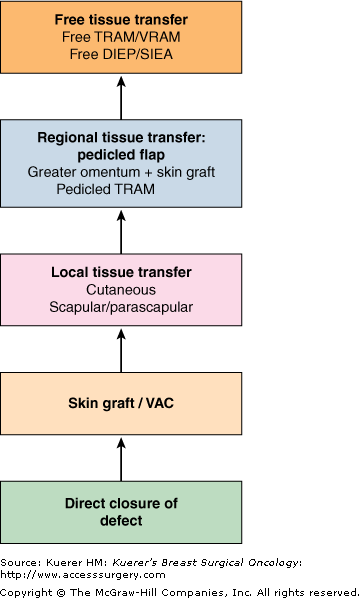
Reconstructive ladder for chest wall defects demonstrating increasing complexity. (DIEP, deep inferior epigastric artery perforator; IGAP, inferior gluteal artery perforator; SGAP, superior gluteal artery perforator; SIEA, superficial inferior epigastric artery perforator; TRAM, transverse rectus abdominis myocutaneous; VAC, vacuum-assisted closure; VRAM, vertical rectus abdominis myocutaneous.)
A skin graft may be an appropriate reconstructive option for defects limited to the skin and subcutaneous tissues. Placed over a greater omental flap, a split-thickness skin graft can be used to resurface the chest wall.42 Likewise, skin grafts may also be used to assist with primary donor site closure after harvesting myocutaneous flaps for chest wall coverage.
The process of engraftment involves revascularization of the skin graft, as the graft initially has no vascular connection and survives via plasma imbibition. The process of revascularization commences approximately 48 hours after graft placement, but graft take may be compromised in a radiated wound bed. This process can be hastened with use of the vacuum-assisted closure (VAC) device (Kinetic Concepts Inc, San Antonio, Texas), which is placed at the time of the initial operation, left in place over the skin graft for 5 days, and then removed at the bedside.43,44 The VAC not only protects the graft in the wound bed but provides a means to improve the adherence of skin grafts in compromised tissues, thus expediting wound closure. Disadvantages of using a skin graft include its propensity to contract and provide a far less aesthetic and durable form of coverage than a vascularized flap.
Various techniques have been used to restore chest wall stability and recreate the chest wall scaffold with the aim of limiting flap movement and consequent paradoxical respiration. The need for a skeletal reconstruction depends on the size and site of the resection; that is, skeletal reconstruction is necessary in cases of removal of the sternum and the anterior and lateral tracts of the ribs, but it may not be necessary for the repair of posterior wall defects entirely covered by the scapula or if the defect can be stabilized by the action of adjacent muscles. While traditionally all sternal defects with greater than 2 adjacent ribs were deemed necessary for rigid stabilization these considerations are evolving. Many authors believe that defects of the sternal and posterior walls need to be stabilized less frequently than anterior or lateral defects.45 Options include the use of autogenous bone grafts, autogenous fascia lata grafts, and numerous synthetic materials, alone or in various combinations.
A spectrum of prosthetic materials may be used for chest wall reconstruction, including metal plates, stainless steel mesh, absorbable mesh (Vicryl mesh, Johnson & Johnson, New Brunswick, New Jersey), nonabsorbable polypropylene mesh (Marlex mesh, Chevron Phillips Chemical, Woodlands, Texas; Prolene mesh, Ethicon, Somerville, New Jersey), and nonporous prosthetic mesh (Gore-Tex mesh; W L. Gore & Associates, Newark, Delaware). The use of synthetic materials is often necessary when reconstructing locally advanced or locally recurrent cancers involving the chest wall (Fig. 68-4). Rigid prosthetic materials, both permanent and absorbable, while less commonly used, may be needed in larger defects (resin plates, methylmethacrylate, and hydroxyapatites combined with tricalcium phosphate).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree